The hybridization of NO3– is sp2 type. Students will learn about how this hybridization occurs and all the steps involved in it. They will also learn about the molecular geometry and the bond angles of nitrate.
| Name of the Molecule | Nitrate |
| Molecular Formula | NO3– |
| Hybridization Type | sp2 |
| Bond Angle | 120o |
| Geometry | Trigonal Planar |
What is the Hybridization of Nitrate?
The easiest way to determine the hybridization of nitrate is by drawing the Lewis structure. After drawing the diagram, we need to count the number of electron pairs and the bonds present in the central nitrogen atom. In NO3– we can see that the central atom is bonded with three oxygen atoms and there are no lone pairs. If we check the Lewis structure further then one of the nitrogen-oxygen bonds is a double bond and two are single bonds.
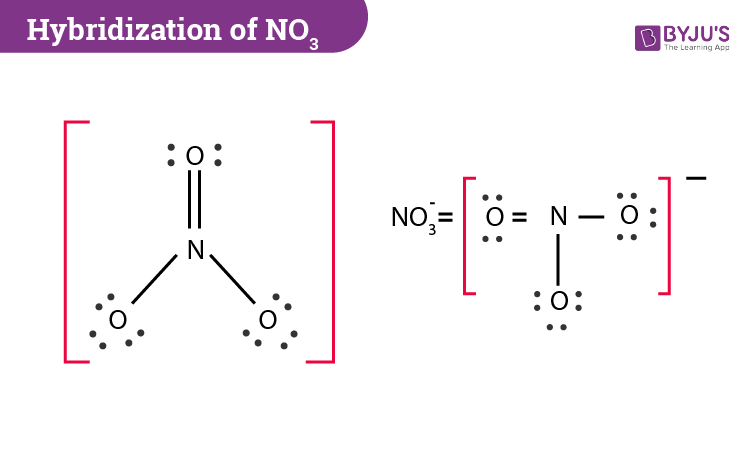
During bonding, nitrogen’s three sp2 orbitals overlap with one s orbital of the oxygen atom. As for the p orbital of nitrogen, it forms a double bond with three oxygen atoms where three pairs of electrons are shared between the p orbital of the nitrogen and one p orbital of each oxygen atoms. The oxygen atoms will also have two p orbitals which will accommodate lone pair of electrons.
Important Points To Remember
- The central atom nitrogen is bonded with three oxygen atoms and there are no lone pairs present.
- Nitrogen’s three sp2 orbitals overlap with one s orbital of the oxygen atom.
- The p orbital of nitrogen forms a double bond with three oxygen atoms.
NO3– Molecular Geometry And Bond Angles
In nitrate, there is one central atom which is surrounded by three identically-bonded oxygen atoms which lie at the corners of a triangle and at the same one-dimensional plane. In essence, nitrate has 3 electron domains and no lone pairs. Therefore, NO3– molecular geometry is slightly bent and is trigonal planar. The bond angle is 120o.

Comments