Hybridization of XeF4 (Xenon Tetrafluoride)
| Name of the Molecule | Xenon Tetrafluoride |
| Molecular Formula | XeF4 |
| Hybridization Type | sp3d2 |
| Bond Angle | 90o or 180o |
| Shape | Square Planar |
What is the Hybridization of Xenon Tetrafluoride?
In xenon tetrafluoride, the hybridization takes place in the central atom which is Xenon (Xe). If we look at the valence shell of Xe there are a total of six electrons in the 5p orbital and two electrons in the 5s orbital. If we observe the 5th shell then there are the d orbital and f orbital in which no electrons are present. In the formation of XeF4, two of the 5p orbital electrons which, in the excited state move to fill the vacant 5 d orbitals. As a result, there are 4 unpaired electrons which include 2 in 5p and 2 in 5d orbitals. This results in sp3d2 hybridization.
In the case of fluorine, four F atoms bond with these four half filled orbitals. These fluorine atoms will then be placed on either side of the central atom.
Important Points To Remember
- The central atom has 6 electron pairs out of which two are lone pair electrons.
- The hybridization in Xenon is sp3 d2 because there is a migration of two electrons of p to d orbital which results in the formation of sigma bond with F.
XeF4 Molecular Geometry And Bond Angles
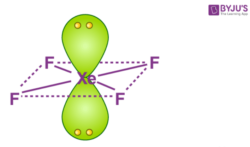
XeF4 consists of two lone pair electrons. Now if we follow the VSEPR theory, the net electronic repulsions has to be minimum. With this, they will acquire a stable state. In order to achieve this, the lone pairs lie in a perpendicular plane in an octahedral arrangement opposite (180 degree) from each other. Therefore, XeF4 molecular geometry is square planar.
Read More About Hybridization of Other Chemical Compounds
- Hybridization Of XeF4
- Hybridization Of SF4
- Hybridization Of PCl3
- Hybridization Of Graphite
- Hybridization Of SO3
Chemical Bonding

Comments