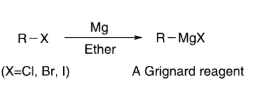
A useful reaction for making alcohols is the Grignard reaction. To make alcohols using Grignard reaction, Grignard Reagent is reacted with a carbonyl compound. Making a Grignard Reagent is fairly simple. The Grignard reaction is used to synthesize carbon-carbon bonds, a key step in creating new molecules for use in academic and industrial applications.
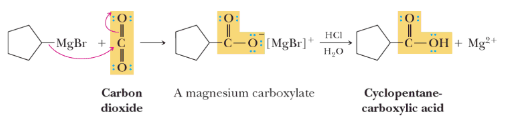
Treating Grignard Reagent with carbon dioxide gives the magnesium salt of a carboxylic acid, which on protonation with aqueous acid, gives a carboxylic acid.
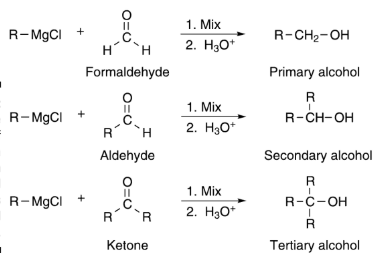
In 1900, in the presence of bromoalkene, Victor Grignard found that magnesium metal had dissolved in ether. The resulting compound, then called the Grignard reagent, has reacted to the formation of new products with different types of molecules (aldehydes or ketones).
This reaction, later called the Grignard reaction, was published in the “Comptes Rendus Hebdomadaires de l’Académie des Sciences” as a communication, and was immediately a hit. Grignard was awarded the PhD title from the University of Lyon in 1901, and the Nobel Prize in Chemistry 11 years later, at the age of 41.
Reactions of Aldehyde and Ketone
This is commonly referred to as a Grignard reagent or an organomagnesium halide such as phenylmagnesium bromide (PhMgBr). Other examples of familiar organometallic compounds include organolithium reactives (RLi) and diorgano cuprates of lithium (R2CuLi, Gilman reactive).
Organometallic compounds are widely used both as stoichiometric reagents and as catalysts to permit basic or complex organic transformations impossible to achieve with conventional organic reagents.
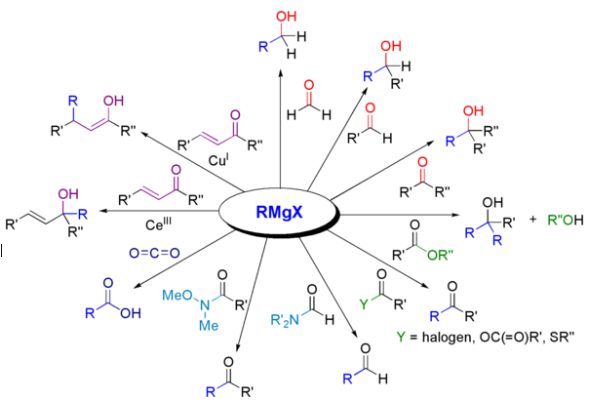
List of Reactions
Unless the conditions are purely anhydrous, the Grignard reactions fail. If only a trace of water or some other source of slightly acidic protons is present, the alkyl halide reaction with the Mg will not even begin. Therefore, all reagents must be dried carefully before beginning the reaction, as must all glassware.
Learn more on NEET Chemistry:
- NEET Chemistry Syllabus
- NEET Chemistry Important Topics
- NEET Chemistry MCQs
- How to Score 160 Plus in NEET Chemistry
- NEET Chemistry Weightage
- Chemistry Formulas for NEET

Comments