According to the CBSE Syllabus 2023-24, this chapter has been renumbered as Chapter 6.
Based on the structure i.e., depending upon the number of halogen atoms in a compound, Alkyl/ Aryl halides are classified as mono, di, and polyhalogen. When compared to carbon, halogen atoms are more electronegative. Therefore
- The bond (carbon-halogen bond) of alkyl halide is polarised.
- Halogen atom carries a partially negative charge
- Carbon atom carries a partially positive charge
Students can refer to the short notes and MCQ questions along with separate solution pdf of this chapter for quick revision from the links below:
- Haloalkanes and Haloarenes Study Notes
- Haloalkanes and Haloarenes MCQ Practice Questions
- Haloalkanes and Haloarenes MCQ Practice Solutions
For more information on SN1 Reaction, watch the below video
CBSE Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes – Related Links
- Haloalkanes and Haloarenes
- Nomenclature Of Haloalkanes And Haloarenes
- SN1 and SN2 Reaction of Haloalkanes
- Haloarenes: Nature of C-X bond
- Reactions of Haloarenes – Electrophilic Substitution Reaction
- Alkyl Halides
Preparation of Alkyl Halides
Alkyl halides are produced by the free radical halogenation of alkanes-
- Step 1 – Adding halogen acids to alkenes
- Step 2 – Replacing –OH group of alcohols with halogens with the use of phosphorus halides or halogen acids, or thionyl chloride
Aryl halides are prepared with the help of electrophilic substitution to arenes. Iodides and Fluorides are prepared with the halogen exchange method. Organohalogens have a higher boiling point when compared to hydrocarbons due to strong van der Waals forces and dipole-dipole forces. They partially dissolve in water but completely dissolve in organic solvents.
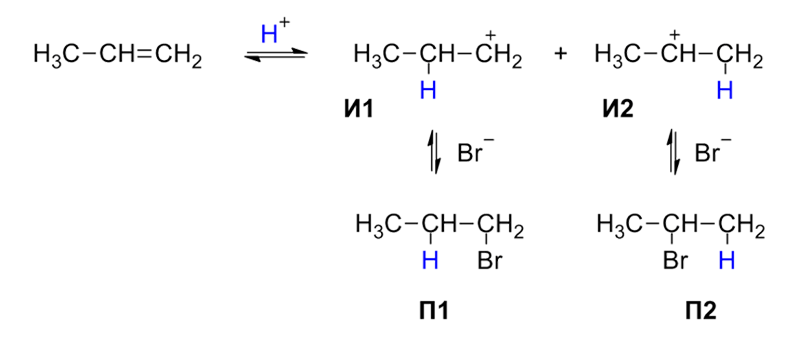
Organometallic compounds are formed by nucleophilic substitution, elimination, and reaction with metal atoms which occurs due to the polarity of a carbon-halogen bond of alkyl halides. Based on the kinetic properties, nucleophilic substitution reactions are classified as SN1 and SN2. Chirality plays very important in SN2 reactions in understanding the reaction mechanisms of these reactions. SN2 reactions are characterized by inversion configuration, whereas SN1 reactions are characterised by racemisation.
For more information on SN1 and SN2 Reactions, watch the below videos
Few Important Questions
- Write the IUPAC names of ClCH2C≡CCH2Br
- Who has the highest dipole moment from the following?
CCl4 and CH2Cl2
- Write the steps to convert Propene to propan-1-ol and Benzyl alcohol to 2-phenylethanoic acid.
- Define ambident nucleophiles. Give an example and explain.
- When n-butyl chloride is treated with alcoholic KOH, what is the result?
Access notes of Haloalkanes and Haloarenes Class 12 Chemistry from BYJU’S to explore more about this chapter. Keep visiting us for the latest updates on CBSE class 12 chemistry notes.
Other Important Links:
| Difference Between Electrophile And Nucleophile | Group 17 Trends Properties |
Frequently Asked Questions on CBSE Class 12 Chemistry Notes Chapter 10: Haloalkanes and Haloarenes
What are the uses of ‘Aldehydes’?
Aldehydes are used in tanning, preserving, and embalming and as a germicide, fungicide, and insecticide for plants, vegetables and the production of certain polymeric materials.
What is nucleophilic addition?
A nucleophilic addition reaction is a chemical addition reaction in which a nucleophile forms a sigma bond with an electron-deficient species.
What is the valency of hydrogen?
Hydrogen has only one valence electron and can form only one bond with an atom that has an incomplete outer shell. Hence the valency of hydrogen is one.
Comments