
Benzene is highly prone to electrophilic substitution reactions compared to addition reactions as it loses its aromaticity during addition reactions. As benzene contains delocalized electrons spanning over carbon atoms in the ring, it is highly attractive to electrophiles and is also highly stable to electrophilic substitutions. Generally, the electrophilic substitution reaction of benzene is a three-step process involving:
- Generation of the electrophile.
- Intermediate carbocation formation.
- Removal of a proton from carbocation intermediate.
Common benzene reactions are
Nitration of Benzene
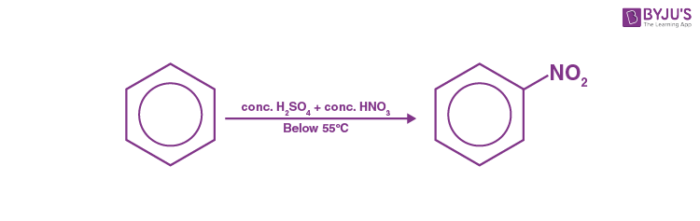
Benzene reacts with concentrated nitric acid at 323-333K in the presence of concentrated sulphuric acid to form nitrobenzene. This reaction is known as nitration of benzene.
Nitration of nitrobenzene
The mechanism for nitration of benzene:
Step 1: Nitric acid accepts a proton from sulphuric acid and then dissociates to form nitronium ion.


Step 2: The nitronium ion acts as an electrophile in the process which further reacts with benzene to form an arenium ion.

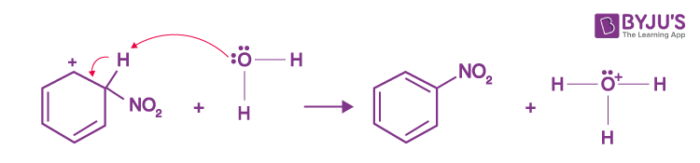
Step 3: The arenium ion then loses its proton to Lewis base forming nitrobenzene.
Sulfonation of Benzene
Sulfonation of benzene is a process of heating benzene with fuming sulphuric acid (H2SO4 +SO3) to produce benzenesulfonic acid. The reaction is reversible in nature.

The mechanism for Sulfonation of benzene
Due to higher electronegativity, oxygen present in sulphuric acid pulls an electron towards itself, generating an electrophile. This attacks the benzene ring, leading to the formation of benzenesulfonic acid.

Halogenation of Benzene
Benzene reacts with halogens in the presence of Lewis acid like FeCl3, FeBr3 to form aryl halides. This reaction is termed as halogenation of benzene.

Halogenation of benzene
The mechanism for halogenation of benzene:
Step 1: Being a Lewis acid, FeBr3 helps in the generation of electrophile bromine ion by combining with the attacking reagent.

Generation of bromine ion
Step 2: The bromine ion acts as an electrophile in the process which further reacts with benzene to form arenium ion which finally converts to bromobenzene.

For detail discussions on nitration, sulfonation, and halogenation of benzene, please visit BYJU’S.
Read more:

Comments