Calcium hydroxide, commonly referred to as slaked lime, is described by the chemical formula Ca(OH)2. It is an inorganic compound which has a white, powdery appearance in its solid-state. However, Ca(OH)2 has a colourless appearance in its crystalline form.
The alternate names of this compound include hydrated lime, slack lime, pickling lime, and caustic lime. Generally, calcium hydroxide is prepared by mixing water and calcium oxide (also known as quick lime).
The chemical reaction between sodium hydroxide and calcium chloride dissolved in water (aqueous CaCl2) also yields this compound. The structure of a Ca(OH)2 molecule is illustrated below.
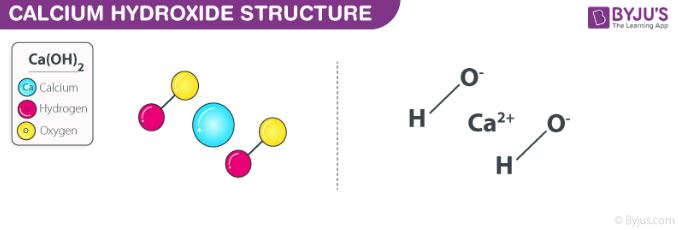
Molecules of calcium hydroxide are held together via ionic bonds between the calcium ion (Ca2+) and two hydroxide ions (OH–). Unprotected exposure to this compound can prove dangerous to humans, leading to irritation of the skin and chemical burns. Exposure to concentrated Ca(OH)2 can lead to lung damage and even blindness.
Table of Contents
- Properties of Calcium Hydroxide
- Physical Properties
- Chemical Properties
- Uses of Calcium Hydroxide
- Frequently Asked Questions
Properties of Calcium Hydroxide
Some important properties of calcium hydroxide are tabulated below.
| Chemical Formula | Ca(OH)2 |
| IUPAC name | Calcium Hydroxide |
| Molecular Weight/Molar Mass | 74.093 grams per mole |
| Density | 2.211 grams per cubic centimetre |
| Appearance | White powder or colourless crystal |
| Melting Point | 853 K |
Physical Properties
- Ca(OH)2 has a hexagonal crystal structure.
- It is not very soluble in water and its solubility reduces with an increase in temperature. For example, its solubility at 0oC is 1.89 g/L and its solubility is 1.73 g/L at 20o C
- At temperatures approaching its melting point, this compound tends to lose water and decompose.
- The solubility product (Ksp) of calcium hydroxide is 5.5 * 10-6.
Chemical Properties
- Ca(OH)2 is quite soluble in glycerol and acids, but only slightly soluble in water. When dissolved in water to a saturation point, it yields a solution which acts as a moderate base (called limewater).
- Limewater reacts with acids and forms salts.
- The saturated solution of calcium hydroxide in water also reacts with and dissolves metals such as aluminium.
- It reacts with carbon dioxide to form calcium carbonate (CaCO3). This reaction is commonly referred to as carbonatation.
Uses of Calcium Hydroxide
- In the process of sewage treatment, calcium hydroxide is used as a clarifying agent or as a flocculant.
- Ca(OH)2 is used in the paper industry during the Kraft process of converting wood into wood pulp.
- It is a very important compound in the preparation of ammonia.
- This compound is also used as a pH modifier due to its basicity.
- The pickling of cucumbers is generally done with the help of Ca(OH)2
- The production of many plastics involves the use of calcium hydroxide as an ingredient.
- It is also used in pesticides, hair care products, and the manufacture of ebonite.
- In root canal procedures, this compound is used to fill the cavities in the human teeth.
- Sugar beets and sugarcane are processed via carbonation, which involves the use of Ca(OH)2.
- Calcium hydroxide is used in the leather industry to separate the fur/hair from the animal hide.
Frequently Asked Questions
What are the uses of calcium hydroxide?
Calcium hydroxide is a white powder which does not have any characteristic odour. It is used in industrial settings such as the treatment of waste, the manufacturing of paper, building and processing of food. There are also several medical and dental applications of this compound. For instance, fillings used in root canal treatments often contain calcium hydroxide.
Is calcium hydroxide acidic or basic?
Calcium hydroxide, also known as slaked lime (with the chemical formula Ca(OH)2) is a source of hydroxide ions when dissolved in aqueous solutions. Therefore, this compound is a base. Due to electrolyte dissociation, this compound liberates OH- ions.
How can calcium hydroxide be prepared?
Calcium hydroxide is formed by the action of water on calcium oxide, also called slaked lime, Ca(OH)2. A small proportion of it dissolves when combined with water, forming a solution known as limewater, the remainder remaining in a suspension called lime milk.
Thus, the structure and the important properties of calcium hydroxide are discussed along with some of its uses. To learn more about this compound and other calcium compounds, such as CaCO3, register with BYJU’S and download the mobile application on your smartphone.

I need past papers for grade 12
Click here to learn more about
CBSE Sample Papers for Class 12
CBSE Class 12 Previous Year Question Papers with Solution