What is Caustic Soda?
Caustic soda is an alkali salt which is also called Lye. It is the common name of sodium hydroxide. This name is given due to the corrosive nature of this salt on animal and plant tissues. It has a wide range of applications. The chemical formula of sodium hydroxide is NaOH.

Preparation of Caustic Soda
Sodium hydroxide can be prepared by various methods like:
- Castner-Kellner process
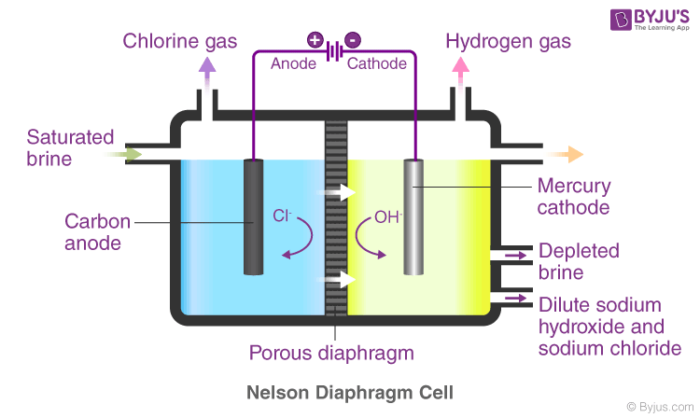
- Nelson Diaphragm cell
- Loewig’s process
Castner-Kellner process
Principle: In the Castner-Kellner method, electrolysis of brine solution is performed in order to obtain sodium hydroxide.
Castner-Kellner cell: It is a steel tank that is rectangular. Ebonite is lined inside the tank. Titanium acts as an anode and a layer of mercury at the bottom of the tank acts as the cathode.
Ionization of brine solution occurs according to the following reaction:
2NaCl→2Na++2Cl−
When the brine solution comes in contact with electric current, ionization takes place. As a result positive and the negative ions move towards the electrodes. Sodium ions get deposited at the mercury cathode forming a sodium amalgam. Chlorine ions move towards the anode and exit the cell from the top.
Reaction at the anode:
2Cl−→Cl2+2e−
Reaction at the cathode:
2Na++2e−→2Na
Formation of NaOH
The amalgam formed is then transferred to another chamber called denuder. In the denuder, it is treated with water to obtain a sodium hydroxide solution. On evaporation of the solution, solid sodium hydroxide is formed. This is a very efficient process in order to obtain pure caustic soda.
Properties of Caustic Soda
- It is a white solid which has a melting point of 591K
- It is a stable compound.
- NaOH is bitter and has a soapy feel to it.
- It is highly soluble in water and moderately soluble in alcohol.
- Sodium hydroxide is strongly alkaline in nature.
Caustic Soda Uses
- It is used as a cleansing agent and in the manufacturing of washing soda.
- Sometimes, sodium hydroxide is also used as a reagent in the laboratories.
- It is used in the preparation of soda lime.
- It is used in the extraction of aluminium by purifying bauxite.
To learn more about Caustic Soda, Register with BYJU’S and download the mobile application on your smartphone.
Frequently asked questions
1. List any two main uses of Caustic Soda.
Ans: Caustic soda is used in the following:
- In the manufacturing of petroleum products.
- It is used in the manufacturing of pulp and paper.
2. Write the chemical formula for caustic soda.
Ans: NaOH is the chemical formula of caustic soda.
3. Caustic soda removes rust. State true or false.
Ans: False. Caustic soda does not remove rust.
4. What is the role of caustic soda in the industrial cleaning process?
Ans: Since caustic soda dissolves the grease, protein-based deposits, fats, and oil, it is added to water and heated, to use it in the cleaning of process equipment, and storage tanks.
5. What are the health effects of caustic soda?
Ans: NaOH is highly corrosive and strongly irritating. It causes severe burns.

Why is NaOH formed at the cathode and not at anode as it is negative?
Na+ (from NaCl) and H+(from water) tends to move to negative terminal (i.e cathode) while Cl- (from NaCl) and OH-(from water) tends to move to positive terminal(i.e anode). Now only one in cathode from Na+ and H+ can be reduced, which has better potential for normal reduction.
sodium hydroxide is a base but caustic soda is a salt. how? sodium hydroxide a base or salt?
NaOH, or sodium hydroxide, is a compound. A compound is classified as either an acid, base, or salt. All bases contain OH- (hydroxide) ions, while all acids contain H+ (hydrogen) ions.