Osmosis Definition
Osmosis is the diffusion of a solvent through a differentially permeable membrane. In biological systems, the solvent will usually be water. Osmosis will occur whenever the water concentrations are different on either side of a differentially permeable membrane.
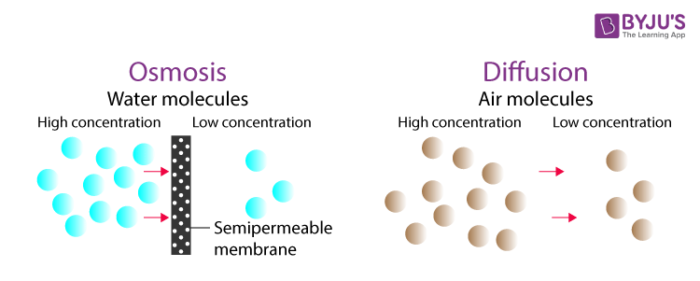
Osmosis can be defined as the movement of water molecules from a higher water concentration area to the area of less water concentration through a semipermeable membrane. In other words, it can be defined as the diffusion of water molecules through a semipermeable membrane. It is a special case of diffusion of water (High to low). For example, water in the roots of plants is transported through osmosis.
What is Diffusion?
Diffusion is the net movement of molecules of a substance from a region of their higher concentration to a region of their lower concentration. Net movement means there are more molecules moving in one direction than in the opposite direction.
Example: Opening a bottle of perfume in a room will result in the gradual diffusion of the perfume from the region of higher concentration (the bottle) out into the room. Diffusion will continue until the perfume has a more or less uniform concentration throughout the bottle and room.
The passive movement of matter; the spontaneous tendency of a substance to move down its concentration, pressure, or temperature gradient. Matter essentially moves (or diffuses) from an area of higher free energy to an area of lower free energy until chemical equilibrium is achieved. In simple words, the movement of matter from high concentration area to low concentration area. For example, perfume diffuses into the air spreading the aroma.
Even though the process of diffusion and osmosis are almost similar, there are some notable differences between them. Few key differences are listed below.
Difference between diffusion and osmosis:
| Osmosis | Diffusion |
| It happens only in the liquid state. | It occurs in all states of matter i.e., solids, liquids or gases. |
| It should be movement of only water or solvent through semipermeable membrane from lower concentration to higher concentration. | Any type of substance that moves from higher concentration area to lower concentration area. |
| It is applied only for the solvent part of the solution. | Diffusion is applied to all states of matter.
|
| It requires semipermeable membrane. | This phenomenon does not require semipermeable membrane. |
| Osmosis depends on the rate of reduction of free energy of one solvent. | It only depends on the free energy of the substance. |
| Influenced by the solute potential | Not influenced by solute potential |
| It is opposed by hydrostatic or turgor pressure. | Hydrostatic pressure or turgor pressure will not occur in diffusion. |
| In this process, concentration of solvent is not equalized. | Concentration of whole substance will be equalized. |
| Factors like solute potential, water potential and pressure potential will not affect osmosis. | Factors like solute potential, water potential and pressure potential will not affect diffusion. |
Importance of Diffusion and Osmosis
Diffusion is essential for many organisms as it is a feature of a number of processes which control and supply vital substances to the body in order for basic survival. A few of these are discussed below. Gas exchange is one of these processes.
- It is when much-needed oxygen is obtained by the body in order for respiration to take place and the waste CO2 is taken out of the body. In us mammals, the exchange takes place in the lungs which contain many alveoli.
- The importance of osmosis is to keep an equilibrium between the outside and inside environment.
- For example, from the soil to the root hairs of the plant for the osmosis of water. The Soil has more concentration of water, therefore, it moves from soil to a region of low concentration (ie. Plant)
Recommended Videos
Simple Diffusion
Read more:

IT IS VERY USEFUL FOR UNDERSTANDING.