Examples of bases are sodium hydroxide, calcium carbonate and potassium oxide. A base is a substance that can neutralize the acid by reacting with hydrogen ions. Most bases are minerals that react with acids to form water and salts. Bases include the oxides, hydroxides and carbonates of metals.
The soluble bases are called alkalis. Sodium hydroxide is an alkali. Copper(ll) oxide is insoluble in water, so it is a base but not an alkali. Therefore, All alkalis are bases, but not all bases are alkalis. Bases have a slippery feel and taste bitter. Bases are defined as proton (H+) acceptors. Common examples of bases include metal oxides and metal hydroxides and ammonium hydroxide.
Table of Contents
What is a Base?
The ionic compounds that produce negative hydroxide (OH−) ions when dissolved in water are called bases. A compound containing negative nonmetal ion as well as a positive metal ion that is held together by the ionic bond is called an ionic compound.
But what are ions? Ions are atoms which become charged particles as a result of losing or gaining electrons. NaOH (sodium hydroxide) is an example of a base. When it dissolves in water, it generates negative hydroxide (OH−) ions and positive sodium (Na+) ions. It can be represented by the following equation:
NaOH →H2O + OH− + Na+
Types of Bases
-
- Strong base – It is a compound that has an ability to remove a proton from a very weak acid. Or they completely dissociate into its ions when in water. Examples are potassium hydroxide (KOH), sodium hydroxide (NaOH).
- Weak base – There is incomplete dissociation when in water. The aqueous solution contains both the weak base as well as its conjugate acid. Examples are ammonia (NH3), water (H2O), pyridine (C5H5N).
- Superbase – These bases are better at deprotonation when compared to a strong base. These have very weak conjugate acids. They can be obtained by mixing an alkali metal with its conjugate acid. It can’t sustain in aqueous solution as it is a stronger base than hydroxide ion. Examples are sodium hydride (NaH), ortho-diethynylbenzene dianion (C6H4(C2)2)2−
- Neutral base – It forms a bond with a neutral acid share an electron pair.
- Solid base – It is active in solid form. Examples are silicon dioxide and sodium hydroxide mounted on alumina.
Some of the examples of bases are given below.
1. Rubidium Hydroxide (RbOH)
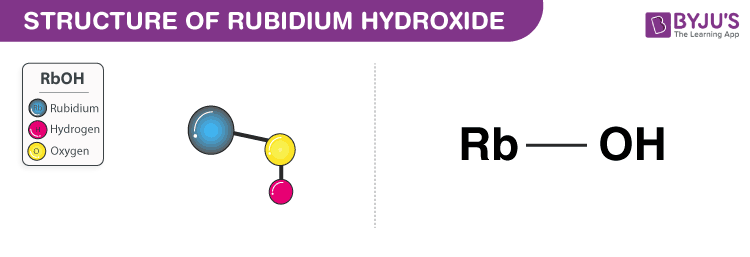
- Rubidium hydroxide is a strong base.
- It appears as a greyish white solid and has a formula RbOH.
- It is also known as rubidium hydrate.
- It is prepared in a lab as it does not occur naturally.
- It has a molecular mass of 102.475 g/mol and density of 3.2 g/cm³.
- The boiling point is 1,390 °C melting point is 301 °C.
- It is highly corrosive.
- When comes in contact with skin causes burns.
- It is used in scientific research.
2. Zinc Hydroxide Zn(OH)2
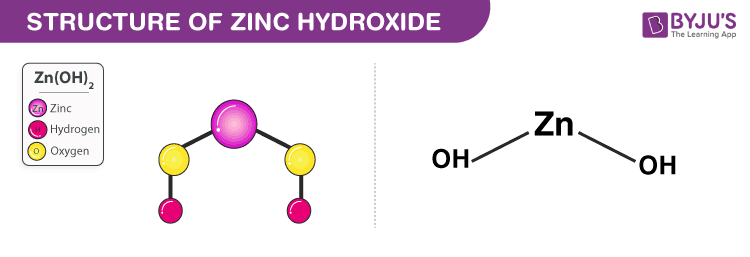
- Zinc hydroxide is a weak base.
- It appears as a white powder and has a chemical formula Zn(OH)2.
- It occurs naturally and can also be prepared in the lab.
- It can be obtained by adding sodium hydroxide to a zinc salt solution.
- It has a molecular mass of 99.424 g/mol and density of 3.053 g/cm³.
- It has a melting point of 125 °C.
- It is used surgical dressings as an absorbent.
Properties of Base
- Aqueous base solution dissociates into ions to conduct electricity.
- It has a pH value greater than 7.
- They form salts on reacting with acids.
- They help in promoting certain chemical reactions.
- They are bitter to taste if placed in alkali solutions.
- Strong or concentrated bases are caustic.
- It changes the indicator colour from red litmus paper to blue litmus paper.
- It has the ability to accept protons from proton donors.
- It contains OH− ions.
- They vigorously react when in contact with acids.
- They are slippery to touch.
- They conduct electricity when dissolved in water.
Since the 17th century acids and bases marked and defined for the first time, their definition has been refined over the decades to reflect an enhanced knowledge of their chemical characteristics. This module presents acid/base chemistry fundamentals, including responses to neutralization.
Frequently Asked Questions – FAQs
What are 5 examples of bases?
Some common strong Arrhenius bases include Potassium hydroxide (KOH), Sodium hydroxide (NaOH), Caesium hydroxide (CsOH), Strontium hydroxide (Sr(OH)2), and Lithium hydroxide (LiOH).
Can base neutralize bases?
A weak base is used to neutralise acids. The taste of bases is bitter or astringent, and they have a pH of greater than 7. Sodium hydroxide, potassium hydroxide, and ammonium hydroxide are common bases. A weak acid is used to neutralise bases.
What is the taste of base?
Bases have a harsh flavour and are less commonly found in foods than acids. Many bases, such as soaps, are slick to touch. Indicators’ colours are also changed by bases.
How can you distinguish an acid from a base without an indicator?
They’re sticky, taste sour, have a sickly sweet smell, like soured milk. They have a pH of less than 7.0 and produce hydrogen gas and salt in reaction to metal.
What is importance of base?
Since numerous reactions and industrial operations produce acidic waste products, bases’ capacity to neutralise acids is extremely helpful. Here are some instances of how bases can be used to neutralise harmful acids: Basic minerals, such as limestone, can neutralise acid rain.
To learn more about examples of bases download BYJU’S the learning app.

This is cool
Thank you so much this really helped