IR spectroscopy (which is short for infrared spectroscopy) deals with the infrared region of the electromagnetic spectrum, i.e. light having a longer wavelength and a lower frequency than visible light. Infrared Spectroscopy generally refers to the analysis of the interaction of a molecule with infrared light.
The IR spectroscopy concept can generally be analyzed in three ways: by measuring reflection, emission, and absorption. The major use of infrared spectroscopy is to determine the functional groups of molecules, relevant to both organic and inorganic chemistry.
Table of Contents
- What is IR Spectroscopy?
- Samples in Infrared Spectroscopy
- IR Spectroscopy Instrumentation
- Frequently Asked Questions – FAQs
What is IR Spectroscopy?
An IR spectrum is essentially a graph plotted with the infrared light absorbed on the Y-axis against. frequency or wavelength on the X-axis. An illustration highlighting the different regions that light can be classified into is given below.
IR Spectroscopy detects frequencies of infrared light that are absorbed by a molecule. Molecules tend to absorb these specific frequencies of light since they correspond to the frequency of the vibration of bonds in the molecule.
The energy required to excite the bonds belonging to a molecule, and to make them vibrate with more amplitude, occurs in the Infrared region. A bond will only interact with the electromagnetic infrared radiation, however, if it is polar.
The presence of separate areas of partial positive and negative charge in a molecule allows the electric field component of the electromagnetic wave to excite the vibrational energy of the molecule.
The change in the vibrational energy leads to another corresponding change in the dipole moment of the given molecule. The intensity of the absorption depends on the polarity of the bond. Symmetrical non-polar bonds in N≡N and O=O do not absorb radiation, as they cannot interact with an electric field.
Check ⇒ NMR Spectroscopy
Regions of the Infrared spectrum
Most of the bands that indicate what functional group is present are found in the region from 4000 cm-1 to 1300 cm-1. Their bands can be identified and used to determine the functional group of an unknown compound.

Bands that are unique to each molecule, similar to a fingerprint, are found in the fingerprint region, from 1300 cm-1 to 400 cm-1. These bands are only used to compare the spectra of one compound to another.
Samples in Infrared Spectroscopy
The samples used in IR spectroscopy can be either in the solid, liquid, or gaseous state.
- Solid samples can be prepared by crushing the sample with a mulling agent which has an oily texture. A thin layer of this mull can now be applied on a salt plate to be measured.
- Liquid samples are generally kept between two salt plates and measured since the plates are transparent to IR light. Salt plates can be made up of sodium chloride, calcium fluoride, or even potassium bromide.
- Since the concentration of gaseous samples can be in parts per million, the sample cell must have a relatively long pathlength, i.e. light must travel for a relatively long distance in the sample cell.
Thus, samples of multiple physical states can be used in Infrared Spectroscopy.
Principle Of Infrared Spectroscopy
The IR spectroscopy theory utilizes the concept that molecules tend to absorb specific frequencies of light that are characteristic of the corresponding structure of the molecules. The energies are reliant on the shape of the molecular surfaces, the associated vibronic coupling, and the mass corresponding to the atoms.
For instance, the molecule can absorb the energy contained in the incident light and the result is a faster rotation or a more pronounced vibration.
IR Spectroscopy Instrumentation
The instrumentation of infrared spectroscopy is illustrated below. First, a beam of IR light from the source is split into two and passed through the reference and the sample respectively.
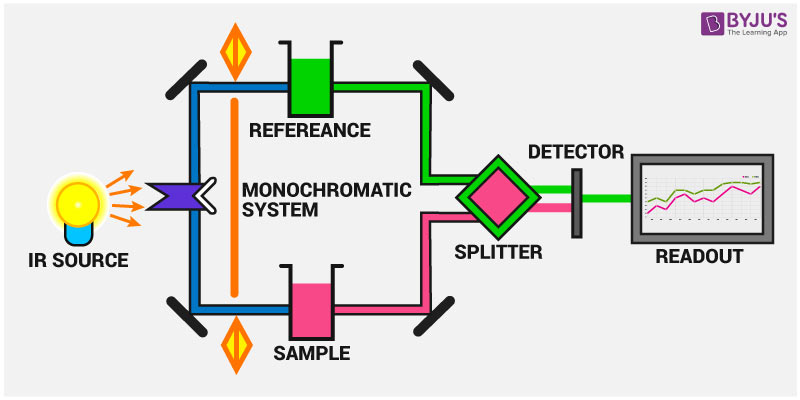
Now, both of these beams are reflected to pass through a splitter and then through a detector. Finally, the required reading is printed out after the processor deciphers the data passed through the detector.
Graph of the IR spectrum
Given below is a sample of typical Infrared Absorption Frequencies.

Thus, IR spectroscopy involves the collection of absorption information and its analysis in the form of a spectrum.
Frequently Asked Questions – FAQs
Can we use water in IR spectroscopy?
Because water has two high infrared absorption peaks, it cannot be employed as a solvent for IR spectroscopy. Also, water is a polar solvent that dissolves alkali halide disks, which are extensively employed in IR.
How sensitive is IR spectroscopy?
Infrared spectroscopy can now identify samples as small as 1 to 10 grams. Almost all organic and certain inorganic molecules can be analysed using infrared spectroscopy. It can be used in both qualitative and quantitative analysis and has a wide range of applications.
What is necessary condition for IR spectroscopy?
The change in the electric dipole moment of the functional group present in a molecule or a sample during the vibration based on the selection rule for IR transitions is a necessary requirement for a molecule or sample to show infrared spectrum.
Which lamp is used in IR spectroscopy?
For infrared spectroscopy, a Globar is employed as a thermal light source. It’s a silicon carbide rod with a diameter of 5 to 10 mm and a length of 20 to 50 mm that’s been electrically heated to 1,000 to 1,650°C (1,830 to 3,000 degrees Fahrenheit).
Which solvent are used in IR spectroscopy?
Carbon Tetrachloride (CCl4) and Carbon Disulfide (CD) are the most prevalent solvents (CS2). Solvents for polar materials include chloroform, methylene chloride, acetonitrile, and acetone. Solids reduced to fine particles can be analysed as a thin paste or mull.
To learn more about similar topics, download the free BYJU’s app from the Google Play store.

can you tell me how IR spectroscopy is useful for pollution control
Infrared spectroscopy is one of many common analytical methods used for the detection and measurement of atmospheric pollution. Much of the fundamental research on atmospheric pollution used spectroscopic techniques, as this technology helps us to comprehend various threats to both public health and the environment.
how can you distinguish alkana and alkene by IR spectroscopy?
Stretching vibrations of the –C=C–H bond are of higher frequency (higher wavenumber) than those of the –C–C–H bond in alkanes. This is a very useful tool for interpreting IR spectra. Only alkenes and aromatics show a C-H stretch slightly higher than 3000 cm-1. Compounds that do not have a C=C bond show C-H stretches only below 3000 cm-1.
Why we are using ccl4 as a solvent
Carbon tetra chloride is used as a solvent in IR spectroscopy because it absorbs at a shallow frequency below 1600 cm−1. Moreover, carbon tetrachloride is a non-polar solvent, i.e. it does interfere with the absorption spectra.