Introduction
Potassium chromate is an inorganic compound with the formula K2CrO4. A yellow crystalline solid is potassium chromate. It’s water-soluble. The main hazard is the environmental danger. Immediate measures to restrict its spread to the setting should be taken. It is used as a fungicide in chemical analysis, making pigments for paints and inks, and creating other compounds of chromium.
Potassium chromate substance is a recognized human cancer and is linked with an enhanced danger of developing sinonasal cavity lung cancer and cancer. Upon heating, it undergoes decomposition and emits toxic fumes of potassium oxide.
Table of Contents
- What is Potassium Chromate?
- Potassium Chromate Structure – K2CrO4
- Properties of Potassium Chromate – K2CrO4
- K2CrO4 Uses (Potassium Chromate)
- Potassium Chromate Indicator
- Frequently Asked Questions – FAQs
What is Potassium Chromate?
Potassium chromate is an inorganic compound with the chemical formula K2CrO4. It is also known as dipotassium monochromate or bipotassium chromate. It appears as a crystalline solid which is yellow in colour. It has no smell and has a disagreeable bitter taste. When potassium chromate is heated it emits chromium fumes that are toxic.
When you come in contact with this inorganic compound, it can affect your throat, lungs, nose, causing ulcerations, bronchitis, shortness of breath, asthma, and pneumonia. It can also affect your kidneys, liver, immune system and gastrointestinal tract. It consists of chromate and potassium ions in a ratio of 1:2. It is a widely used laboratory chemical. It is a strong oxidising agent and is highly corrosive.
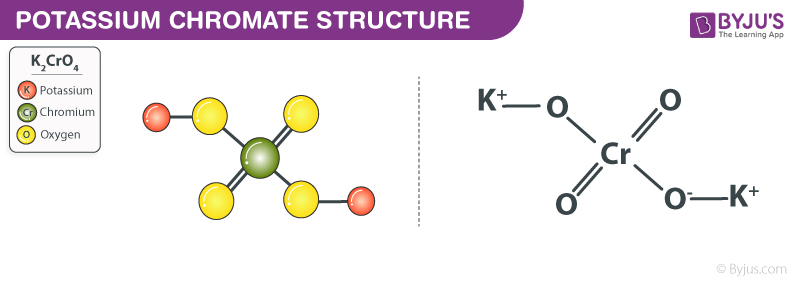
Potassium Chromate Structure – K2CrO4
Potassium chromate consists of two positively charged potassium cations (K+ ions) and one chromate anion (CrO42- ion). The chromate anion is made up of one chromium atom and four oxygen atoms. In this polyatomic ion, the central chromium atom is doubly bonded to two oxygen atoms and singly bonded to two oxygen atoms. The singly bonded oxygen atoms hold a charge of -1 (which enables them to obtain complete octet configurations).
Properties of Potassium Chromate – K2CrO4
| K2CrO4 | Potassium chromate |
| Molecular Weight/ Molar Mass | 194.1896 g/mol |
| Density | 2.7320 g/cm³ |
| Boiling Point | 1,000°C |
| Melting Point | 968°C |
A few other important properties of potassium chromate are listed below.
- This compound has a yellow, powdery appearance and is odourless.
- At a temperature of 20 degrees celsius, potassium chromate has a solubility of 629 grams per litre in water.
- When the temperature is increased to 100 degrees celsius, the solubility of this compound in water increases to 792 grams per litre.
- K2CrO4 crystallizes in a rhombic lattice.
Potassium chromate can be produced by treating potassium dichromate along with potassium hydroxide. The most common oxidation state of chromium is +2, +3 and +6 and the electronic configuration of chromium 3d54s1 and the atomic number is 24. Potassium chromate is a powerful oxidizing agent and is extremely corrosive. This material is used in the production of colourants and in procedures of textile dyeing. The chemical formula for potassium chromate is K2CrO4.
K2CrO4 Uses (Potassium Chromate)
The uses of potassium chromate are listed below.
- It is used as an oxidising agent during organic synthesis.
- It is used in qualitative inorganic analysis to detect the ions in aqueous solution.
- It is used in the manufacture of dyes.
- It is used in precipitation titrations as an indicator.
- It is used in the textile dyeing process.
- It is used in making inks and pigments for paints.
- It is used as a fungicide.
- It is used to make several other chromium compounds.
- It is used in the leaching process.
Potassium chromate is a known human carcinogen and it is associated with an increased risk to develop cancer of the sinonasal cavity and lung cancer.
Potassium Chromate Indicator
Potassium chromate is a metalochrome indicator. Quantitative estimation of chloride, bromide and cyanide ions by titrating with a standard solution of silver nitrate with a few ml of potassium chromate as an indicator forms the basis of Mohr’s method. At the equivalence point, the yellow-coloured solution of potassium chromate changes to a brick-red precipitate of silver chromate.
When potassium chromate is used as an indicator, after the extinction of chloride ions, silver ions react with chromate ions and solubility product of silver chromate is exceeded and it begins to form a reddish-brown precipitate
In precipitation titration a known volume of halide solution taken in a conical flask to which 1-2ml of potassium chromate indicator is added, and titrated against a standard solution of silver nitrate taken in the burette. The indicator forms a red-coloured precipitate of silver chromate at the endpoint with the first drop of excess silver nitrate added.
Frequently Asked Questions – FAQs
Is potassium chromate aqueous?
A yellow crystalline solid is the potassium chromate. It’s water-soluble. The primary danger is an environmental threat. Immediate steps to minimize their spread to the ecosystem should be taken.
Is potassium chromate an acid or base?
Potassium chromate, commonly referred to as tarapacaite, is an inorganic compound at room temperature that is a yellow orthorhombic or hexagonal crystal. It is a conjugate base of chromic acid. The absolute density is 2,732 g/cm3, and 968 ° C is the melting point. It is poisonous and can dissolve into water to form a solution for hydrolysis of alkaline chromate ions.
What is the colour of potassium chromate?
Yellow. Potassium chromate is the chromate anion’s yellow base of potassium chloride. It is a growing chemical in the laboratory, while industrial sodium chromate is essential.
Is potassium chromate hazardous?
Potassium chromate is highly toxic to aquatic organisms and may cause long-term aquatic environmental effects.
Why is Potassium chromate used as an indicator?
Potassium Chromate is used as an indicator with a regular silver nitrate solution to assess chloride by titration.
Learn more about the chemical behaviour and importance of K2CrO4 from the expert faculties at BYJU’S.

Comments