What is a Neutralization Reaction?
A neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products.
In a neutralization reaction, there is a combination of H+ ions and OH– ions which form water. A neutralisation reaction is generally an acid-base neutralization reaction.
Table of Contents
- Relation Between the Strength of Reactants and Resultant pH
- Neutralization Reaction Equation
- Application
- Frequently Asked Questions – FAQs
Neutralization Reaction – Acid-Base Reaction to form Salt and Water
Relation Between the Strength of Reactants and Resultant pH
Depending upon the strength of the constituent acids and bases the pH of the products varies.
| Strength of Acid | Strength of Base | Resultant pH |
|---|---|---|
| Strong | Strong | 7 |
| Strong | Weak | < 7 |
| Weak | Strong | > 7 |
| Weak | Weak | If,
Ka>Kb => pH < 7 Ka=Kb => pH = 7 Ka<Kb => pH > 7 |
Neutralization Reaction Equation
acid + base(alkali) → salt + water
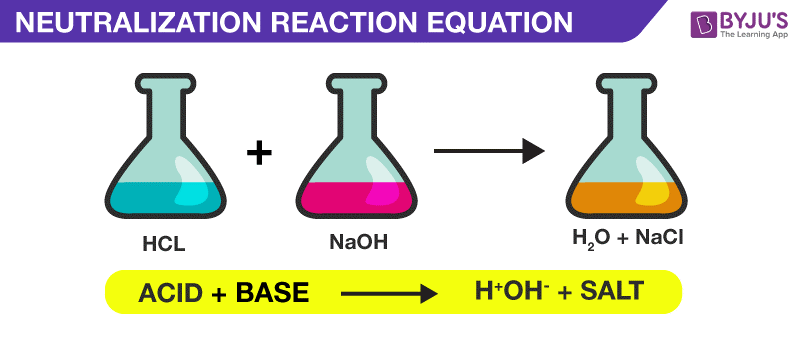
Neutralization Reaction Equation
Neutralization Reaction Examples
- Formation of Sodium Chloride (Common Salt):
HCl + NaOH → NaCl + H2O - Titration methods using phenolphthalein.
Application of Neutralization Reaction
1. Titration methods:
The method of chemical titration is employed to find unknown concentrations of acids or bases by finding their neutralization point. To find the point where the neutralization happens, we use a pH indicator or pH meter. With the help of simple stoichiometric calculations and knowledge of the volume and molarity of the known quantity, the molarity of the unknown particle can be found out.
2. Wastewater treatment:
Most of the waste that comes in the form of industrial effluents have their fair share of toxicity which will be harmful to our environment. Thus, we need to neutralize their toxicity before they can be thrown out. Based on the application, different chemicals are used. Some common examples are Sodium Bicarbonate, Magnesium Hydroxide, Calcium Oxide, Calcium Carbonate.
3. Nanomaterial synthesis:
To facilitate the chemical reduction of metal precursors, the heat of a neutralization reaction is used.
4. In our digestive systems:
When food is moved between our stomach and intestines, the food needs to be neutralized. But for nutrients to be absorbed through the intestine walls an alkaline environment is required. Antacid bicarbonate is produced to create this favourable condition.
5. Controlling soil pH:
For optimal plant growth in any soil, there are certain conditions which are required. Some examples of materials mixed in the soil to neutralize it from acidity are:
- Calcium Carbonate (Limestone)
- Calcium Hydroxide(Slaked lime)
Recommended Video
Neutralisation Reactions

Frequently Asked Questions – FAQs
What is one example of a neutralization reaction?
A neutral ionic compound is a salt. Let’s see how both water and salt are created by a neutralisation reaction, using the reaction between hydrochloric acid solutions and sodium hydroxide as an example.
What is the general end product of a neutralization reaction?
Net neutralization reactions of ionic equations include solid bases, solid salts, water, and solid acids. The reaction between an acid and a base that forms water and salt is neutralisation. Solid acids, solid bases, solid salts, and water can provide net ionic equations for neutralisation reactions.
What is the use of neutralization?
For the neutralisation of acidic soils, farmers use lime (calcium oxide). There is hydrochloric acid in the gut, and so much of this induces indigestion. To neutralise the excess acid, antacid tablets contain bases such as magnesium hydroxide and magnesium carbonate.
In what way you can neutralize an acid?
A weak base is used to neutralise acids. Bases have a flavour that is bitter or astringent and have a pH greater than 7. Sodium hydroxide, potassium hydroxide and ammonium hydroxide are widely used.
What is the neutralization reaction used in daily life?
To treat wasp stings that are alkaline in nature, vinegar is used. To treat bee stings and ant bites that are acidic in nature, baking powder is used. Toothpaste contains bases that neutralise the acid that our mouth creates from bacteria. To make the cakes grow, baking powder is typically used.
To learn more about the different types of chemical reactions like decomposition reaction and more, register with BYJU’S.
Read more:


Mind blowing answers
Thank you so much! This is an AWESOME document and each and every part was clear.