Para nitroacetanilide is also called 4-Nitroacetanilide, para nitroacetanilide is a chemical compound that is a nitroacetanilide derivative prepared from acetanilide and nitrating mixture. Along with para product a trace of ortho product is also formed.
Table of Contents
Aim:
To prepare p-Nitroacetanilide from acetanilide and acetic acid in the presence of nitrating mixture.
Theory:
The organic compound p-nitroacetanilide is prepared from acetanilide through nitration. When acetanilide is treated with nitrating mixture that is a mixture of nitric acid and sulphuric acid p-nitroacetanilide is formed. Along with p-nitroacetanilide, o-nitroacetanilide is also formed as a minor product. Since o-nitroacetanilide is very much soluble in alcohol it is very easy to isolate p-nitroacetanilide through crystallization.
| Also Read: Preparation of p-Nitroacetanilide Viva Questions |
The chemical reactions involved in this process is given below.
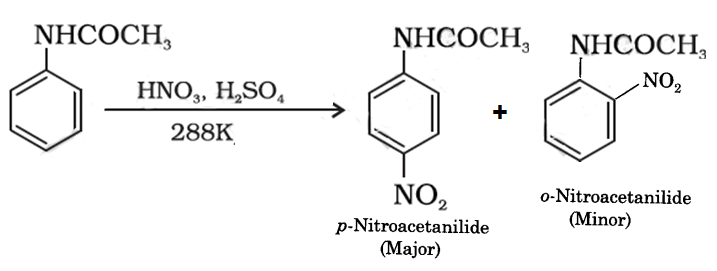
It is an electrophilic substitution reaction. The electrophile -NO2 will attach the para position because the -NHCOCH3 is an electron releasing group. Nitro anilines can be prepared by this type of reactions because nitration of aniline is not possible, amino group gets oxidized with nitrating mixture. In order to protect the amino group from oxidation acetanilide is first nitrated to give p-nitroacetanilide and then on hydrolysis to give p-nitroaniline which is difficult to obtain by direct nitration.
Other names – N-(4-nitrophenyl) acetamide, p-Acetamidonitrobenzene, p-Nitroacetanilide, N-Acetyl-4-nitroaniline
Materials Required:
-
-
-
- Acetanilide
- Acetic acid
- Concentrated Sulphuric acid
- Fuming Nitric acid
- Ethyl alcohol
- Conical flask
- Beaker
- Dropping funnel
- Filter paper
- Glass rod
- Buchner funnel
- Pipette
-
-
Apparatus Setup:
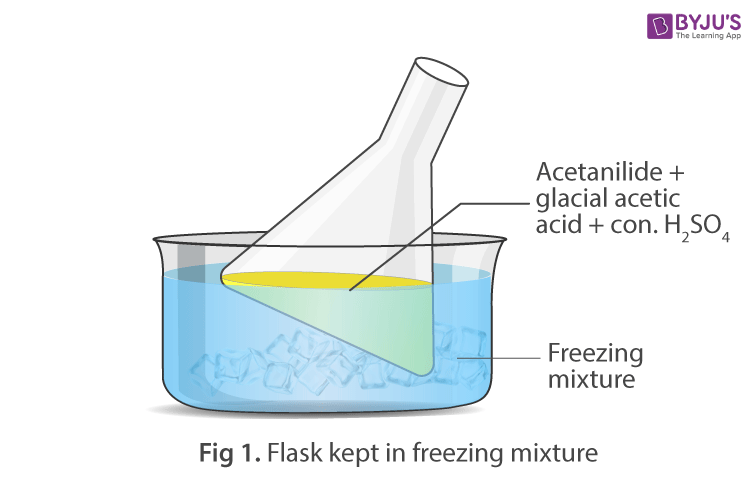
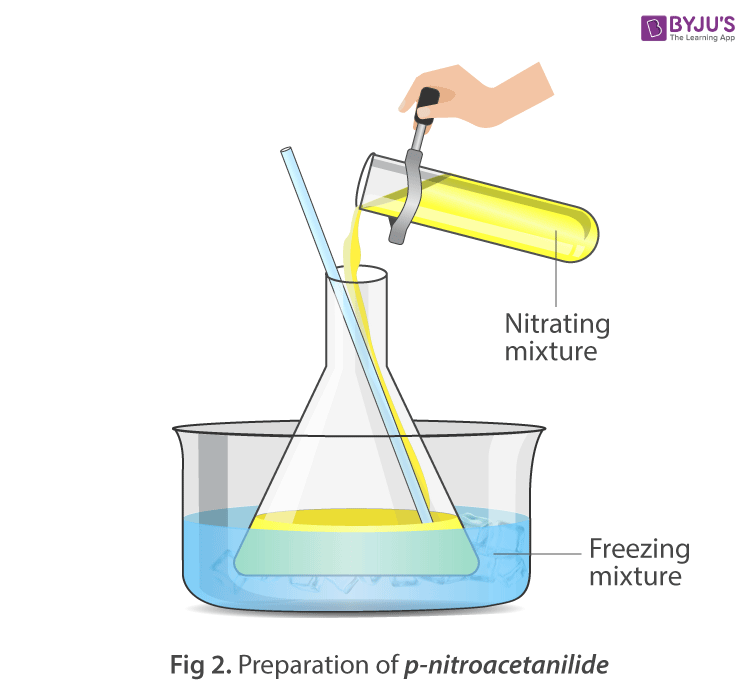
Procedure:
-
-
-
- Take 3gm of finely powdered acetanilide in a clean beaker and dissolve it by adding glacial acetic acid by stirring the content carefully at room temperature.
- Gently warm the mixture to dissolve acetanilide completely.
- Cool the solution and add concentrated sulphuric acid slowly with constant stirring. The solution becomes warm, keep the mixture in ice-bath and clear solution is obtained.
- To the cool solution add fuming nitric acid dropwise through a dropping funnel with constant stirring.
- Maintain the temperature below 20oC during the whole process.
- Once the addition of nitric acid is completed, the beaker is removed from the freezing mixture bath and allowed to stand for half an hour at room temperature.
- Pour the mixture into 100gm of crushed ice in a beaker and stir well.
- Large crystals of p-nitroacetanilide are obtained. Filter the crystals through filter paper.
- The separated p-nitroacetanilide is washed with cold water in order to remove excess of acid.
- It is crystallized from ethyl alcohol. Dry the crystals in the folds of filter paper and weigh them to know the yield.
-
-
Observations:
| Colour of the crystals | Colourless |
| Melting point | 214oC |
| Expected yield | 4gm |
Results and Discussion:
The yield of p-Nitroacetanilide is _____gm.
Precautions:
-
-
-
- Temperature should not exceed more than 20oC.
- Advisable to add nitric acid into the reaction mixture while it is immersed in ice-bath
- Add fuming nitric acid drop by drop carefully and do not inhale the fumes of nitric acid.
-
-
Keep visiting BYJU’S to learn more about class 12 CBSE chemistry practicals.
Frequently Asked Questions on Preparation of p-Nitroacetanilide
How p-nitroacetanilide is separated from o-nitroacetanilide in the crude sample?
o-nitroacetanilide is more soluble in ethanol so that it remains in the mother liquor and during crystallization only p-nitroacetanilide separates out.
What is called nitrating mixture?
The combination of concentrated sulphuric acid and fuming nitric acid is called nitrating mixture.
What is the formula for p-nitroacetanilide? Give its IUPAC name.
The formula for p-nitroacetanilide is C8H8N2O3, and the IUPAC name is N-(4-nitrophenyl)acetamide.
Why is the ortho derivative minor and para product is major? Give reason.
This reaction is electrophilic substitution reaction and the nitronium ion formed is directed towards ortho and para positions. Due to steric hindrance in ortho position the nitronium electrophile is directed more towards para position. Therefore, para product is major.
Mention the uses of p-nitroacetanilide.
It is used in pharmaceuticals in preparation of paracetamol and phenacetin. Used in pesticides and rubber chemicals also used as an intermediate for dyes.

What is the role of H2SO4 which added to the beaker after glacial acetic acid?
p-Nitroaniline is prepared from p-nitro acetanilide due to hydrolysis of acetate ion from acetamido functional group in presence of concentrated sulphuric acid.
Click here to learn about the Preparation of p-Nitroacetanilide