What is Glucose?
Glucose is a group of carbohydrates which is a simple sugar with a chemical formula C6H12O6. It is made of six carbon atoms and an aldehyde group. Therefore, it is referred to as aldohexose. It exists in two forms viz open-chain (acyclic) form or ring (cyclic) form.
The primary source of energy required for living organisms is glucose. Plants and algae prepare glucose during the process of photosynthesis with the help of water, sunlight, and carbon dioxide. It is naturally found in fruits and honey. Glucose in animals is obtained by the process of glycogenolysis.
Table of Contents
- Recommended Videos
- Steps to Draw Open Chain Structure of a Glucose Molecule
- Steps to Draw a Ring Structure of a Glucose Molecule
- Glucose Properties
- Structure of Fructose
- Open Chain and Ring Structure of a Fructose Molecule
- Glucose and Fructose are Which Isomers
- Frequently Asked Questions – FAQs
Recommended Videos

1. Steps to Draw Open Chain Structure of a Glucose Molecule
Follow the steps given below to draw an acyclic form of glucose.
Step 1: Draw 6 carbon atoms
Step 2: Draw extended arms for all the carbon atoms excluding the first one.
Step 3: Now draw hydrogen to carbon bond such that four are on one side and one on the other side.
Step 4: The remaining spaces should be filled with an OH group. (Important – transpose
(OH) to —> (HO) for the left side to show that the oxygen is bonded to carbon)
Step 5: Complete the ends with two single-bonded hydrogen bond and one double-bonded carbon.
The open chain structure of glucose was given by Baeyer however these structure could not explain why glucose fails to react with Schiff base, sodium bisulphate or the process of mutarotation. Haworth introduced cyclic structure of glucose which confirms the existence of alpha and beta forms of glucose, mutarotation etc.

Open Chain Structure of a Glucose Molecule
2. Steps to Draw a Ring Structure of a Glucose Molecule
Follow the steps given below to draw a cyclic form of glucose.
Step 1: Construct a hexagon
Step 2: Draw carbon atoms at 5 consecutive edges.
Step 3: Attach an oxygen atom at the left out edge.
Step 4: Attach the 4 carbon atoms with H and OH groups.
Step 5: Complete the structure by attaching the left out carbon atom to two hydrogen atoms, OH group, and one carbon atom.

Ring Structure of a Glucose Molecule
Glucose Properties:
| Glucose Formula | C6H12O6 |
| Glucose Molar mass | 180.156 g/mol |
| Glucose Chemical Formula | C6H12O6 |
| IUPAC Name | D-Glucose |
Also, Check ⇒ Amylose
Structure of Fructose
Fructose is a monosaccharide which is a simple sugar with a chemical formula C6H12O6. It is also called as fruit sugar. It was discovered in the year 1847 by a French chemist Augustin-Pierre Dubrunfaut. It consists of a 6-carbon polyhydroxy ketone.
Crystalline fructose occurs as a cyclic six-membered structure delinquent to the stability of the hemiketal and internal hydrogen-bonding. This form is called D-fructopyranose. It mainly occurs in honey, vine fruits, most root vegetables, berries, and flowers. Commercially it can be obtained from sugar beets, maize, and sugar cane.
Open Chain and Ring Structure of a Fructose Molecule
The cyclic structure of fructose is given below:
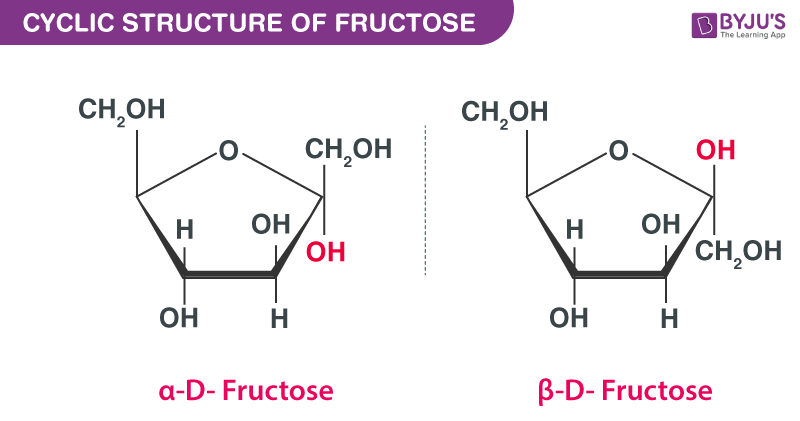
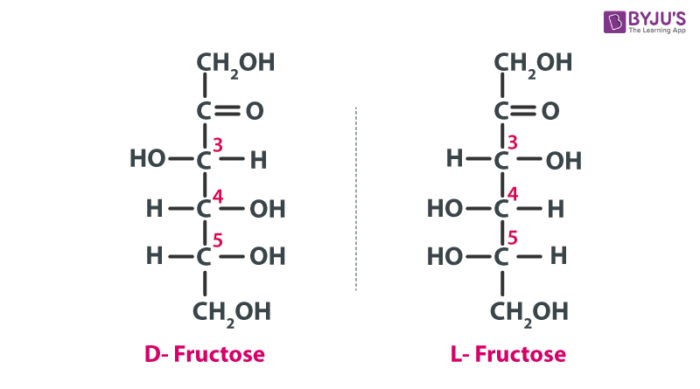
The Fischer projections of D- and L-fructose is given below:
Fructose Properties
Dry fructose appears as a white, crystalline solid which is sweet, and odourless. It is soluble in alcohol, water, and ether.
| Fructose Formula | C6H12O6 |
| Fructose Molar mass | 180.156 g/mol |
| Fructose Chemical Formula | C6H12O6 |
| Density | 1.694 g/cm3 |
| Melting point | 103°C |
Glucose and Fructose are Which Isomers
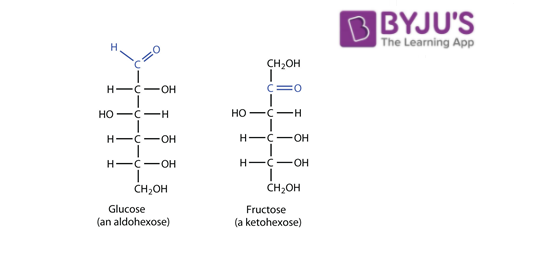
Glucose and fructose are functional isomers of each other because they have same molecular formula that is C6H12O6 But different functional group in their chemical formula. Glucose has aldehyde group while fructose has ketone as functional group. They differ in the nature of the functional group. Glucose is an aldehyde and fructose is a ketone.
Frequently Asked Questions – FAQs
What are the different types of glucose?
The D-isomer, D-glucose, also known as dextrose, is commonly found in nature, but the L-isomer, L-glucose, is not. Hydrolysis of carbohydrates such as dairy sugar (lactose), plant sugar (sacrose), maltose, cellulose, glycogen, etc. can be used to obtain glucose.
What are fructose and glucose?
Both fructose and glucose are simple sugars of monosaccharides. When digested, both starch and sugar, whether sucrose or high-fructose corn syrup (HCFS), produce big quantities of glucose.
Why Glucose is a monosaccharide?
Monosaccharides (mono- = “one ;” sacchar- = “sugar”) are simple sugars, of which glucose is the most prevalent.
Where does glucose come from?
The food we consume comes from glucose or sugar. The prevalent sources of glucose are carbohydrates such as fruit, bread pasta, and cereals. In our stomachs, these foods are split into sugar and then absorbed into the bloodstream.
What is the function of fructose?
Like glucose, fructose provides the cells with energy. Cells process fructose to obtain energy through a method called aerobic breathing, which mainly involves burning fructose in the presence of oxygen to generate ATP, the molecule of cellular energy.

The given cyclic structure of fructose is wrong, see it has 7 carbons.
Wrong
Resolved, updated and corrected the image. Thank you.