Diamagnetic materials are those materials that are freely magnetized when placed in a magnetic field. However, the magnetization is in the direction opposite to that of the magnetic field. The magnetism that is shown by these materials is known as diamagnetism.
Download Complete Chapter Notes of Magnetism and matter
Download Now
We can relate to diamagnetic materials in our daily lives if we think of substances that are non-magnetic. They include substances such as wood, water, some plastics and a few metals as well.
Table of Contents
- What Are Diamagnetic Materials?
- Properties of Diamagnetic Materials
- Superconductors
- Diamagnetic Materials Examples and Demonstration
- Important Points to Remember about Diamagnetism
- Meissner Effect
- Frequently Asked Questions – FAQs
What Are Diamagnetic Materials?
In simple terms, diamagnetic materials are substances that are usually repelled by a magnetic field. Electrons in an atom revolve around the nucleus, and thus possess orbital angular momentum. The resultant magnetic momentum in an atom of the diamagnetic material is zero. Common examples of diamagnetic materials are plastic bodies, petroleum substances, copper, etc.
In diamagnetic materials, there are no atomic dipoles due to the pairing between the electrons. When an external magnetic field is applied, dipoles are induced in the diamagnetic materials in such a way that induced dipoles oppose the external magnetic field according to Lenz’s law.
Thus, all the materials whose atoms contain paired electrons show diamagnetic properties.
Properties of Diamagnetic Materials
1. There are no atomic dipoles in diamagnetic materials because the resultant magnetic moment of each atom is zero due to paired electrons.
2. Diamagnetic materials are repelled by a magnet.
3. The substances are weakly repelled by the field, so in a nonuniform field, these substances have a tendency to move from a strong to a weak part of the external magnetic field.
4. The intensity of magnetization (I) is very small, negative and proportional to the magnetizing field.
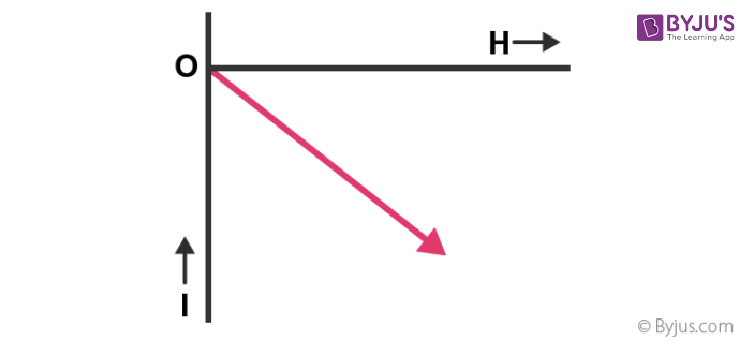
5. Magnetic susceptibility is small and negative.
6. The relative permeability is slightly less than unity.
Also Read: Ferromagnetic Materials
7. Diamagnetic materials are independent of temperature. These materials do not obey Curie’s law.
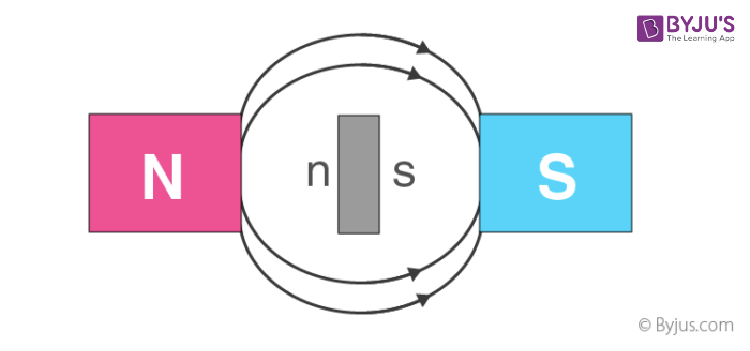
8. A rod of diamagnetic material comes to rest with its length perpendicular to the direction of the field when it is suspended in a uniform magnetic field because the field is strongest at the poles.
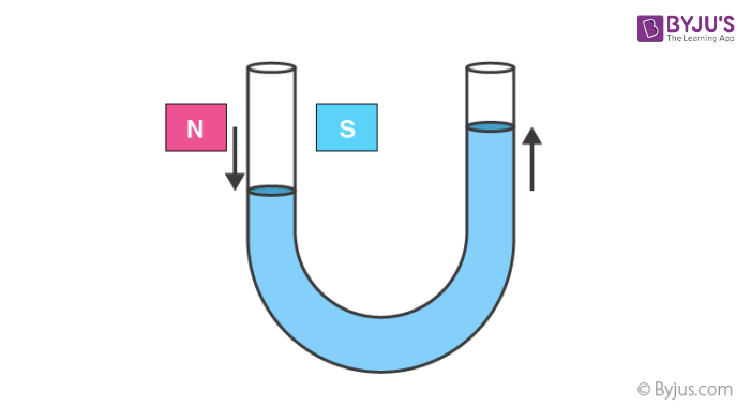
9. A diamagnetic liquid in a U-tube depresses the limb, which is between the poles of a magnet.
10. The magnetic dipole moment is small and opposite to the magnetic field H.
11. If a diamagnetic liquid is placed in a watch glass, placed on two pole pieces that are quite close to each other, then liquid accumulates at the sides and shows depression in the middle where the field is strongest.
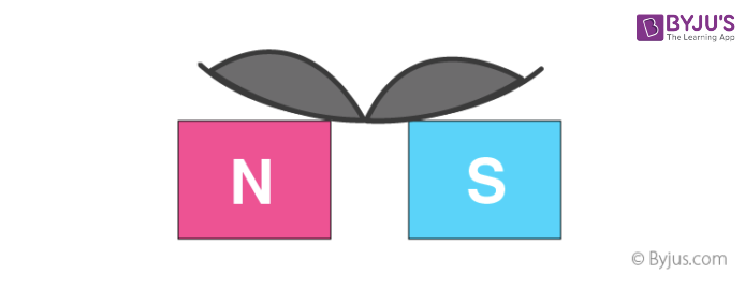
12. If a liquid is placed on a watch glass, placed over two pole pieces that are sufficiently apart (more than in the previous case), then liquid accumulates in the middle where the field is weakest.
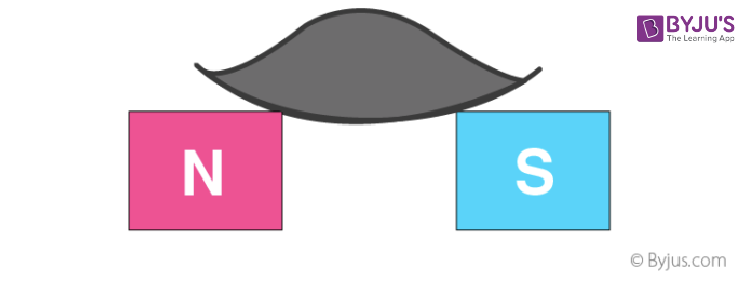
13. The origin of diamagnetism is the induced dipole moment due to a change in the orbital motion of electrons in atoms by the applied field.
Superconductors
Superconductors are basically strong diamagnetic materials that exhibit a volume susceptibility of χ v = − 1 (dimensionless). They obey perfect diamagnetic screening and can be considered perfect diamagnets as they tend to expel all magnetic fields.
Also Read: Paramagnetic Materials
Diamagnetic Materials Examples and Demonstration
Some of the most common examples of diamagnetic substances are Copper, Zinc, Bismuth, Silver, Gold, Antimony, Marble, Water, Glass, NACL, etc.
| Material | χv [× 10−5 (SI units)] |
| Superconductor | −105 |
| Pyrolytic carbon | −40.9 |
| Bismuth | −16.6 |
| Mercury | −2.9 |
| Silver | −2.6 |
| Carbon (diamond) | −2.1 |
| Lead | −1.8 |
| Carbon (graphite) | −1.6 |
| Copper | −1.0 |
| Water | −0.91 |
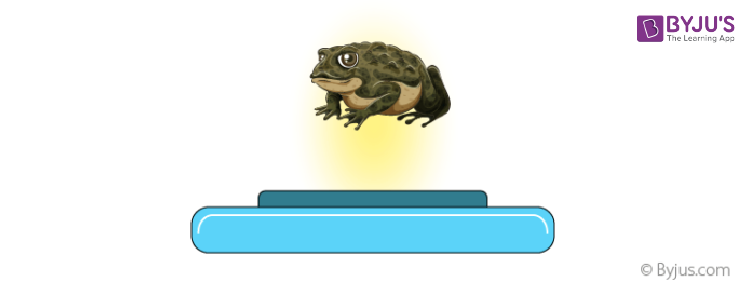
Frog in the Magnetic Field
A frog is levitated in a 15 tesla magnetic field. The levitation force is exerted on the diamagnetic water molecules in the frog’s body. This is the best-known example of diamagnetism.
Important Points to Remember about Diamagnetism
- Diamagnetism is present in all materials and is independent of temperature, but the effect is so weak it is often neglected in comparison to paramagnetism and ferromagnetic effects.
- Diamagnetism is possible in solids, liquids, and gases.
Meissner Effect
If a permanent magnet is brought near a superconductor, superconducting material induces current, which completely opposes the magnetic field applied by the permanent magnet. An applied magnetic field is expelled by the superconductor so that the field is zero in its interior. Thus, a superconductor in the Meissner state behaves like a perfect diamagnet.
Question: A diamagnetic material is heated from 300 K to 650 K. What is the change in its diamagnetic susceptibility?
Answer:
Diamagnetic susceptibility has no dependence on temperature, so heating a material will not change its diamagnetic susceptibility.
Frequently Asked Questions on Diamagnetic Materials
What are diamagnetic materials?
Diamagnetic materials are those that are not attracted to magnets and magnetic fields.
Give a few examples of diamagnetic materials.
Copper, Water, Bismuth, Zinc, Marble, Glass and Gold are a few examples of diamagnetic materials.
What causes negative diamagnetic susceptibility?
There are no total spins in diamagnetic materials since the electron pairs are all together. These materials’ magnetic fields are in the opposite direction as the applied magnetic field. The low negative susceptibility of the diamagnetic indicates that it is diamagnetic.

Comments