Phosphoric acid is one of the most popular acids that is used in many industries, especially in the manufacturing of fertilizers. The salts of this acid which are known as phosphates are used mainly in agriculture and even at home. Students might even hear this term often in their chemistry classes. Therefore, we will learn about this chemical compound and understand various things related to it here on this page.
Phosphoric acid falls into the category of weak acids. It is also referred to as orthophosphoric acid which helps us to easily distinguish it from other phosphoric acids such as polyphosphoric acid. Another name for this acid is phosphoric(V) acid. Phosphoric acid’s formula is written as H3PO4. This acid is a non-toxic acid and in its pure form, it is a solid at room temperature. It has a molar mass of 97.99 g/mol.
H3PO4 is one of the most important and useful mineral acids. The acid is mostly available in the form of an aqueous solution (almost 85%) and is odorless, colorless, and non-volatile liquid. The solution is a sticky liquid and even though this acid is categorized as a weak acid it can still cause irritation or burns in the skin as well as damage to the eyes and membranes in the nose.
| IUPAC Name | Ortho Phosphoric Acid |
| Chemical Formula | H3PO4 |
| Molar Mass | 97.99 g/mol |
| Density | 2.030 g.cm-3 |
| Melting Point | 42.4 °C (108.3 °F; 315.5 K) |
| Boiling Point | 407 °C (765 °F; 680 K) |
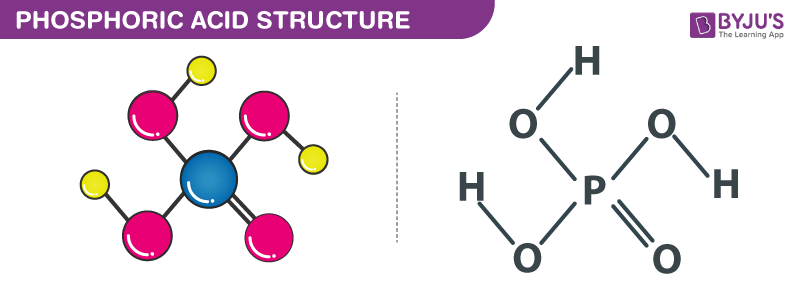
Phosphoric Acid Structure
When we talk about the structure of phosphoric acid, the central phosphorus atom is bonded together with an oxygen atom through a double bond. It is also connected to three hydroxyl (-OH) groups through single bonds.
Phosphoric Acid Properties
Below we will look at the chemical and physical properties of H3PO4.
Physical Properties
- Pure phosphoric acid is normally in the form of a white crystalline solid.
- It has a melting point of 42.4° C.
- The acid is colourless.
- It is also odourless and a viscous liquid with a density of 2.030 g.cm-3.
- H3PO4 is non-toxic and non-volatile.
Chemical Properties
- Phosphoric acid or H3PO4 can release up to three H+ ions. Due to this property, it can react differently in comparison to other mineral acids.
- Reaction with bases usually results in the formation of three classes of salts.
- When the molecules of phosphoric acid are exposed to high temperatures it forms dimers, trimers and even long polymeric chains as seen in poly phosphoric acids and meta-phosphoric acids.
Preparation Of Phosphoric Acid
Phosphoric acid is usually prepared or manufactured using two different processes. These include:
a) The ‘wet’ process.
b) Thermal process.
We will look at the process in detail below:
(a) Wet Process
During the wet process, phosphoric acid is produced from a naturally occurring crystal rock known as fluorapatite which contains the phosphate mineral. This compound is reacted with concentrated sulphuric acid and water. When the reaction takes place it results in the formation of phosphoric acid and calcium sulfate (gypsum) as well as some insoluble impurities. The extra chemical compounds and impurities are removed by the process of filtration and evaporation. The acid is then concentrated to ca 56-70% P2O5 (super phosphoric acid) using vacuum distillation. The reaction can be represented as:
Ca5(PO4)3Cl + 5H2SO4 + 10H2O → 3H3PO4 + 5CaSO4·2H2O + HCl
The product from the ‘wet process’ acid is impure but can be used, without further purification, for fertilizer manufacture.
(b) Thermal process
Another method that is used in obtaining phosphoric acid is the thermal process. In this, phosphorus is heated or burnt at high temperature in the presence of air. The burning results in the generation of phosphorus pentoxide which is then condensed to form a white powder. It is then hydrated in a separate process to obtain phosphoric acid.
Sometimes steam is also added to the burner where a condensed form of polyphosphoric acids is produced. The products are then directly passed into a hydration tower where the gaseous phosphorus oxide is absorbed and phosphoric acid is obtained. Nonetheless, a purer product is obtained in the first process.
Phosphoric Acid Uses
Phosphoric acid is one of the most popular chemical compounds that have several uses in different industries and even in products that we consume. Here are some popular uses of H3PO4.
In Agriculture
One of the most common uses of phosphoric acid is in the agriculture domain. It is widely used in the production of fertilizer and as a flavouring agent in animal or poultry feed.
In Dentistry
It is also used in dentistry where dentists often use the chemical compound as an etching solution and for cleaning the teeth. Phosphoric acid is also found in mouth cleaning products. Alternatively, phosphoric acid is found in anti-nausea medicines.
Treatment of Rust
Phosphoric acid is also used in treating rusts and removing them from metal components. It is used in the process of the phosphate conversion coating. This helps in corrosion resistance.
Skincare Products
Phosphoric acid mostly used in adjusting or controlling the pH level in skincare products. It is used in toothpaste, soaps, and detergents as well.
In The Food And Beverage Industry
Phosphoric acid is often used as a food additive and is mainly utilized to acidify foods and beverages. It helps in creating a certain taste.
Other Applications
- It is used in phosphoric acid fuel cells.
- Production of activated carbon.
- Compound semiconductor processing.
- It is used in sanitizing brewing and dairy industries.
Phosphates
Phosphates are basically the salts of phosphates. Here we will look at three important phosphates of H3PO4.
(a) Ammonium phosphates
Some common phosphate salts include monoammonium dihydrogen phosphate and diammonium hydrogen phosphate. These are prepared by combining anhydrous ammonia with phosphoric acid. They are mostly used as fertilizers.
(b) Calcium phosphates
We have already talked about these above in the preparation of phosphoric acid. However, just to recall calcium phosphates are produced when phosphate rock is reacted with sulphuric acid. This type of phosphate is also called superphosphate.
(c) Sodium phosphates
When phosphoric acid is reacted with a concentrated solution of sodium hydroxide under controlled conditions and proportions sodium phosphates are formed. The product that is generated is in the form of solid crystals. Some examples of sodium phosphates are Monosodium dihydrogen phosphate, Disodium hydrogen phosphate, Trisodium phosphate, Disodium pyrophosphate.
Phosphoric Acid Hazards
Phosphoric acid is mostly non-toxic and does not cause harm to the skin or any part of the body in low concentration. It is dangerous only at higher concentrations and can cause severe skin irritation or burns and even damage to the eyes. H3PO4 can also cause irritation in the respiratory tract if vapours are inhaled. This acid should be stored in a metallic or coated fibreboard container (with polyethylene inner package) and kept in a cool and well-ventilated place.

Comments