Clausius Statement from the second law of thermodynamics states that:
“It is impossible to design a device which works on a cycle and produces no effect other than heat transfer from a cold body to a hot body.”
That is, heat transfer can only occur spontaneously in the direction of temperature decrease. For example, we cannot construct a refrigerator that operates without any work input.
Who was Rudolf Clausius?
Rudolf Julius Emanuel Clausius, a German mathematician and physicist, is one of the central founders of thermodynamics science. There are a few important concepts introduced by Rudolf Clausius one of which is the theory of heat which is a restatement of the Carnot cycle. In the year 1850, he published a paper on “The Moving Force of Heat”. In 1865 he introduced entropy and virial theorem in the year 1870.
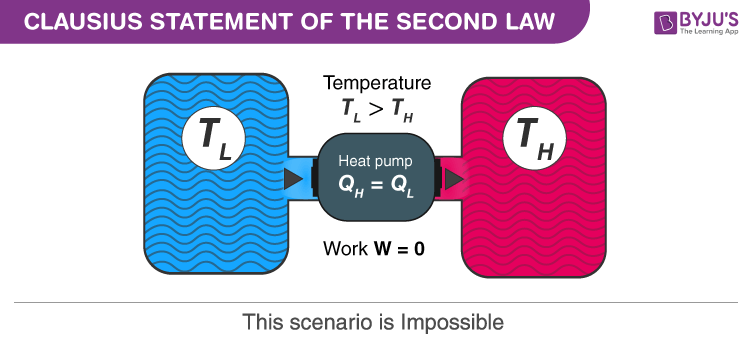
Clausius Statement Example
Example of Clausius statement is a refrigerator with COP (coefficient of performance) equal to infinity which is impossible.
You may also want to check out these topics given below!
- Carnot Engine – Thermodynamic Engine
- Difference Between Air Conditioning and Refrigeration
- Heat Pump And Refrigerator: Applications
- Newton’s Law Of Cooling
If you wish to learn more physics concepts with the help of interactive video lessons, download BYJU’S – The Learning App.
Second Law of Thermodynamics Explained

Comments