What are Aldehydes?
Aldehydes are members of a class of organic chemical compounds represented by the general structural formula R-CHO. R may be hydrogen or a hydrocarbon radical–substituted or unsubstituted.
Many aldehydes are volatile, flammable liquids which at normal room temperature form vapour in explosive concentrations. Fire and explosion precautions must be most rigorous in the case of the lower members of the aldehyde family and safeguards with respect to irritant properties must also be most extensive for the lower members and for those with an unsaturated or substituted chain.
Table of contents
Naturally Occurring Aldehydes
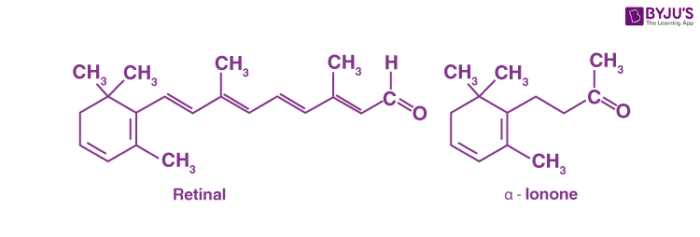
The carbonyl group is the most common functional group in oxygen-containing organic compounds isolated from biological sources. One of the two suffixes in common names may indicate the presence of a carbonyl group in a molecule. If the carbonyl compound is an aldehyde we use the suffix -al. If the carbonyl compound is a ketone we use the suffix -one. For example, retinal is an aldehyde required for vision. The first part of the name indicates that this compound is present in the retina and the suffix tells us that it is an aldehyde. Another example of a common name is alpha ionone, a fragrant ketone responsible for the scent of irises that are used in perfumes.
Carbonyl groups are present in some steroids. For example, the synthetic steroids norethindrone an oral contraceptive and methandrostenolone, an anabolic steroid both contain a carbonyl group.
The structures of naturally occurring aldehyde and ketone are given below.
General Properties of Aldehydes
1. Physical State
Except formaldehyde which is gas at room temperature, most of the common aldehydes and ketones are liquid at ordinary temperature. The lower molecular mass aldehydes have a sharp rather unpleasant smell but higher molecular mass aldehydes and ketones are pleasant smelling. In fact, some ketones are used in perfumery. Some aromatic aldehydes obtained from natural sources have a very pleasant fragrance.
2. Boiling points
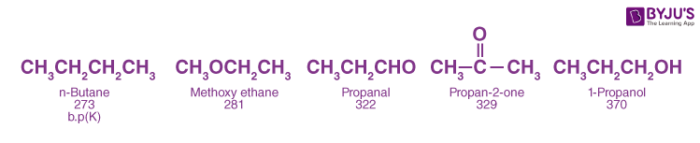
As both aldehydes and ketones contain polar carbonyl groups, there is molecular association due to stronger dipole-dipole interactions between the opposite ends of dipoles. Therefore, the boiling points of aldehydes and ketones are higher than non-polar alkanes and weakly polar ethers of comparable molecular masses. However, the boiling points of aldehydes and ketones are lower than those of alcohols of comparable molecular masses due to the absence of intermolecular hydrogen bonding. For example, the following compounds have a molecular mass around 60 but their boiling points are quite different.
3. Solubility
The lower aldehydes and ketones such as methanal, ethanal and propanone are miscible with water in all proportions as they can form hydrogen bonds with water.
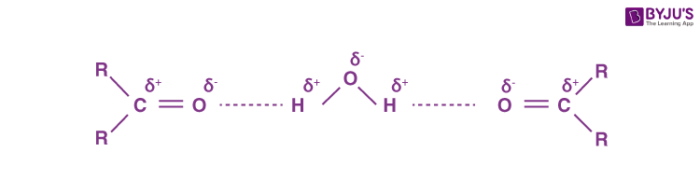
Solubility in water sharply decreases with increase in molecular mass due to increase in the length of non-polar alkyl chain. All aldehydes and ketones are soluble in organic solvents like benzene ether etc.
Uses of Aldehydes
Aldehydes are important intermediates for the manufacture of resins, plasticizers, solvents and dyes. They are used in the textile, food, rubber, plastics, leather, chemical and healthcare industries. The aromatic aldehydes and the higher aliphatic aldehydes are used in the manufacture of perfumes and essences.
Aldehydes are primarily used to manufacture acetic acid but is also used in the manufacture of ethyl acetate, peracetic acid, pyridine derivatives, perfumes, dyes, plastics and synthetic flavouring agents. Formaldehyde has an extremely wide range of uses related to both its solvents and germicidal properties. It is used in plastics production.
Formaldehyde is a powerful antiseptic, germicide, fungicide and preservative used to disinfectant inanimate objects. Benzaldehyde is used in organic synthesis, mainly in the manufacture of rubber accelerators and as a synthetic flavouring agent in foods. It is used in the synthesis of amino acids and in the manufacture of perfumes, flavourings, plasticizers and gasoline additives.
Related Topics
- Preparation of Aldehydes
- Nomenclature of Aldehydes
- Properties of Aldehydes
- Structure of Aldehydes
- Tests for Aldehydes
Frequently Asked Questions on Aldehydes – FAQs
How are aldehydes prepared?
Aldehydes are produced through primary alcohol oxidation. The acidified potassium dichromate(VI) solution used as the oxidizing agent may oxidize the produced aldehyde further to a carboxylic acid. To stop at the aldehyde, you need to keep this from happening.
What are the physical properties of aldehydes?
The carbonyl group’s polarity notably affects the physical properties of melting point and boiling point, solubility, and moment dipole. Hydrocarbons, compounds that consist only of the hydrogen and carbon elements, are essentially nonpolar and therefore have low melting and boiling points.
Why are aldehydes used in perfumes?
Aldehydes are the result of partial oxidation and often derive the moniker given to them from the name of the acid it forms. They are used to make synthetic resins and to create dyes, flavourings, perfumes, and other chemicals. Others are used as preservatives and as disinfectants.
What are natural aldehydes?
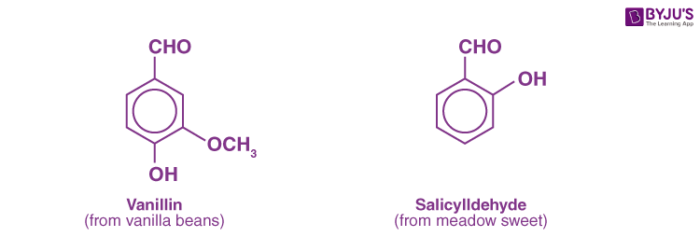
Aldehydes are a family of reactive, organic compounds that can be produced in natural products such as cinnamon bark (cinnamaldehyde) and vanilla bean (vanillin) as well as in laboratories.
Why are aldehydes more reactive than ketones?
Given both steric and electronic effects, aldehydes are usually more reactive to nucleophilic substitutions than ketones. In aldehydes, on one side of the carbonyl group is attached the comparatively small hydrogen atom, while on the other side is attached a larger R group.

Comments