Bravais Lattice refers to the 14 different 3-dimensional configurations into which atoms can be arranged in crystals. The smallest group of symmetrically aligned atoms which can be repeated in an array to make up the entire crystal is called a unit cell.
There are several ways to describe a lattice. The most fundamental description is known as the Bravais lattice. In words, a Bravais lattice is an array of discrete points with an arrangement and orientation that look exactly the same from any of the discrete points, that is the lattice points are indistinguishable from one another.
Thus, a Bravais lattice can refer to one of the 14 different types of unit cells that a crystal structure can be made up of. These lattices are named after the French physicist Auguste Bravais.
Table of Contents
14 Types of Bravais Lattices
Out of 14 types of Bravais lattices some 7 types of Bravais lattices in three-dimensional space are listed in this subsection. Note that the letters a, b, and c have been used to denote the dimensions of the unit cells whereas the letters 𝛂, 𝞫, and 𝝲 denote the corresponding angles in the unit cells.
1. Cubic Systems
In Bravais lattices with cubic systems, the following relationships can be observed.
a = b = c
𝛂 = 𝞫 = 𝝲 = 90o
The 3 possible types of cubic cells have been illustrated below.
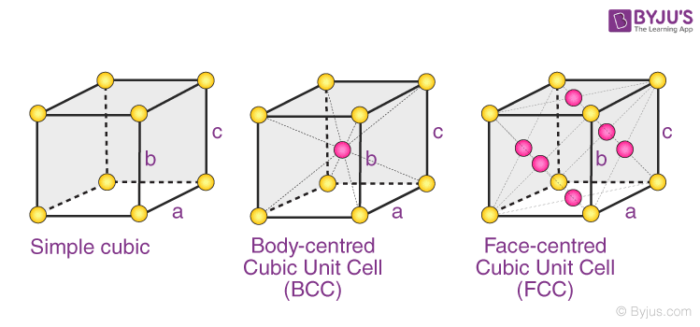
These three possible cubic Bravais lattices are –
- Primitive (or Simple) Cubic Cell (P)
- Body-Centered Cubic Cell (I)
- Face-Centered Cubic Cell (F)
Examples: Polonium has a simple cubic structure, iron has a body-centered cubic structure, and copper has a face-centered cubic structure.
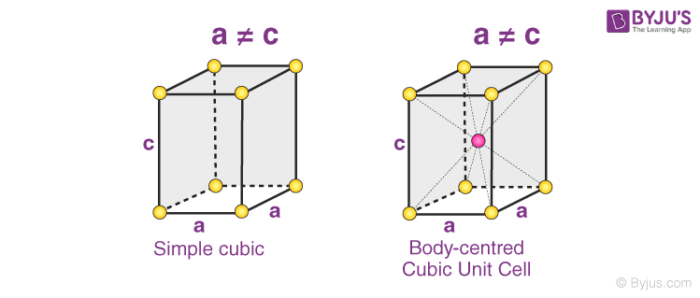
2. Orthorhombic Systems
The Bravais lattices with orthorhombic systems obey the following equations:
a ≠ b ≠ c
𝛂 = 𝞫 = 𝝲 = 90o
The four types of orthorhombic systems (simple, base centered, face-centered, and body-centered orthorhombic cells) are illustrated below.
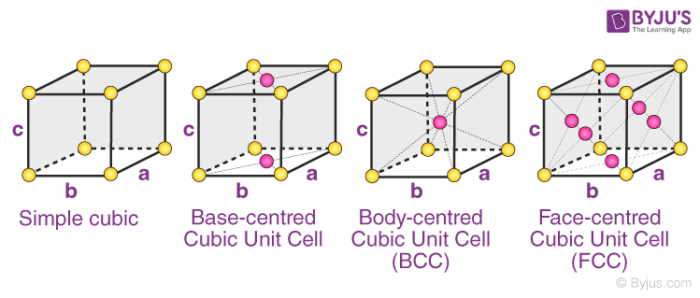
Examples of Orthorhombic Systems:
- Rhombic Sulphur has a simple orthorhombic structure
- Magnesium sulfate heptahydrate (MgSO4.7H2O) is made up of a base centred orthorhombic structure.
- Potassium Nitrate has a structure which is body-centered orthorhombic.
- An example of a substance with a face-centered orthorhombic structure is barium sulfate.
3. Tetragonal Systems
In tetragonal Bravais lattices, the following relations are observed:
a = b ≠ c
𝛂 = 𝞫 = 𝝲 = 90o
The two types of tetragonal systems are simple tetragonal cells and body-centered tetragonal cells, as illustrated below.
Examples of tetragonal Bravais lattices are – stannic oxide (simple tetragonal) and titanium dioxide (body-centered tetragonal)
4. Monoclinic Systems
Bravais lattices having monoclinic systems obey the following relations:
a ≠ b ≠ c
𝞫 = 𝝲 = 90o and 𝛂 ≠ 90o
The two possible types of monoclinic systems are primitive and base centered monoclinic cells, as illustrated below.
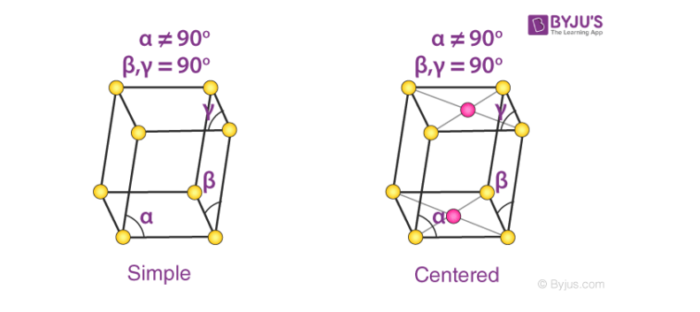
Cubic cells are – Monoclinic sulphur (simple monoclinic) and sodium sulfate decahydrate (base centered monoclinic)
5. Triclinic System
There exists only one type of triclinic Bravais lattice, which is a primitive cell. It obeys the following relationship.
a ≠ b ≠ c
𝛂 ≠ 𝞫 ≠ 𝝲 ≠ 90o
An illustration of a simple triclinic cell is given below.
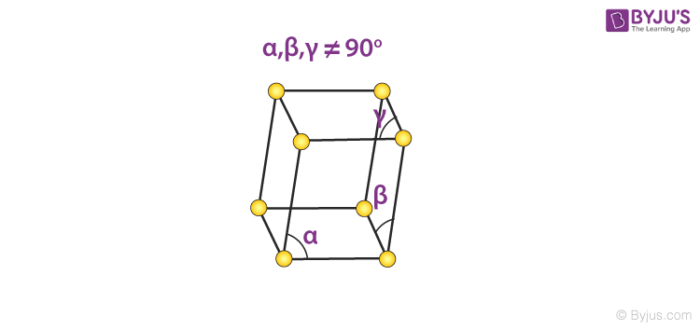
Such unit cells are found in the structure of potassium dichromate (Chemical formula K2Cr2O7).
6. Rhombohedral System
Only the primitive unit cell for a rhombohedral system exists. Its cell relation is given by:
a = b = c
𝛂 = 𝞫 = 𝝲 ≠ 90o
An illustration of the primitive rhombohedral cell is provided below.
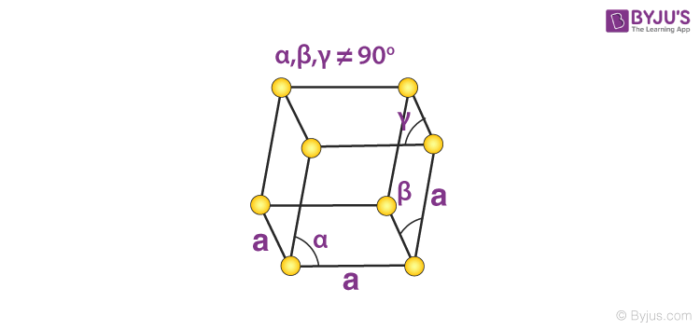
Calcite and sodium nitrate are made up of simple rhombohedral unit cells.
7. Hexagonal System
The only type of hexagonal Bravais lattice is the simple hexagonal cell. It has the following relations between cell sides and angles.
a = b ≠ c
𝛂 = 𝞫 = 90o and 𝝲 = 120o
An illustration of a simple hexagonal cell is provided below.
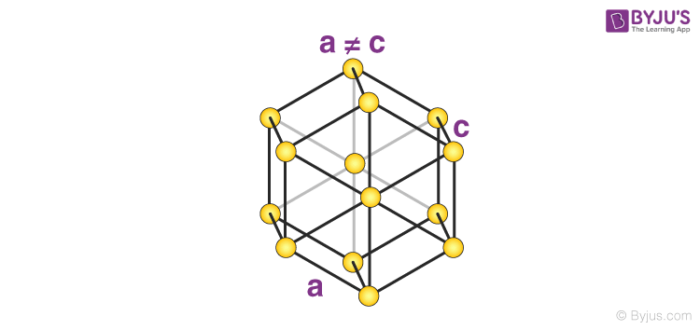
Zinc oxide and beryllium oxide are made up of simple hexagonal unit cells.
Thus, it can be noted that all 14 possible Bravais lattices differ in their cell length and angle relationships. It is important to keep in mind that the Bravais lattice is not always the same as the crystal lattice.
Frequently Asked Questions – FAQs
What is Bravais and non Bravais lattice?
All lattice points in a Bravais lattice are equivalent, hence all atoms in the crystal must be of the same sort. In a non-Bravais lattice, however, some of the lattice points are non-equivalent.
Why are there only 14 Bravais lattices?
Because there are only 14 different ways to choose nonequivalent basis vectors in 3-space, and using these basis vectors, 14 different spacial lattice types can be generated.
What is the difference between space lattice and Bravais lattice?
The 3D lattice is commonly referred to as a space lattice. There are 14 different types of space lattices when they are categorised by their space group. These are termed 14 Bravais lattices. They should be referred to as 14 different space groups of lattices.
Why pentagonal lattice is not possible?
The length, borders of primary axes, and angle between unit cells are all lattice constants. Although crystals appear to have 5-fold symmetry, such symmetry is not achievable. Crystals can only occur in rotational axes of 2, 3, 4, or 6.
What is lattice site?
Each atom, molecule, or ion (constituent particle) in a crystal lattice is represented by a single point. These points are termed lattice site or lattice point. Lattice sites or points together are joined by a straight line in a crystal lattice.

Thanks it realy help me
Thanks it really helps me a lot in GATE
Thanks 👍 it really helps me a lot