What are Alkynes?
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon-carbon triple bond.
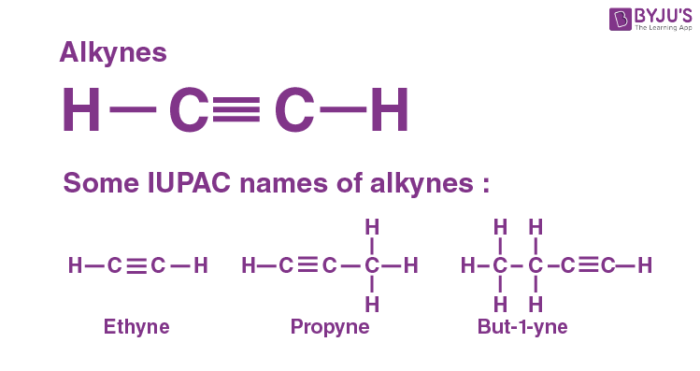
The alkynes are unsaturated hydrocarbons that contain one triple bond, the general formula of alkynes CnH2n-2 and the triple bond is known as the ‘acetylenic bond’. Many alkynes have been found in nature. Ethyne (C2H2) is the first member of the alkyne family, with two carbon atoms connected by a triple bond.
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon-carbon triple bond. Like other hydrocarbons, alkynes are generally hydrophobic. Ethyne is more commonly known under the trivial name acetylene. It is the simplest of the alkynes, consisting of two carbon atoms connected by a triple bond, leaving each carbon able to bond to one hydrogen atom.
Related Topics
- Preparation of Alkynes
- Properties of Alkynes
- Triple Bond in Alkynes
- Acidity of Alkynes
- IUPAC Nomenclature of Alkynes
Recommended Videos
Isomerism in Alkynes
Alkynes show three types of isomerism
- Chain isomerism
- Position isomerism
- Functional isomerism
1. Chain isomerism
It is due to the different arrangement of carbon atoms in the chain that is straight-chain or branched.
Example: 4-methylpent-2-yne and hex-2-yne

2. Position isomerism
It is due to the difference in the location of the triple bond
Example: Pent-1-yne and pent-2-yne

3. Functional isomerism
Alkynes are isomeric with alkadienes both being represented by the general formula CnH2n-2.
Example: But-1-yne and buta-1,3-diene

The triple bond present in alkynes is the functional group for alkynes. The property of alkynes is largely determined by the triple bond. In ethyne, the triple bonded carbon atoms exhibit sp hybridisation. Therefore the ethylene molecule is a linear molecule.
Alkynes Homologous Series
A homologous series is a collection of carbon compounds in which the same functional group replaces the hydrogen atom. Owing to the addition of the same type of functional group in the chain, these compounds have similar chemical properties.
A homologous series is a group of hydrocarbons that share the same general formula and have similar chemical properties. They are organic compounds with structural and functional groups that are identical. The homologous series’ constituents exhibit a gradation of physical properties.
The first ten carbon straight chain alkynes’ molecular formulas and names are tabulated below.
| Name | Molecular Formula |
| Ethyne | C2H2 |
| Propyne | C3H4 |
| 1-Butyne | C4H6 |
| 1-Pentyne | C5H8 |
| 1-Hexyne | C6H10 |
| 1-Heptyne | C7H12 |
| 1-Octyne | C8H14 |
| 1-Nonyne | C9H16 |
| 1-Decyne | C10H18 |
The linear or straight geometry is a primary characteristic of alkynes with a carbon-carbon triple bond. A part of the molecule is in a single-dimensional straight line. Ethyne is used to make a variety of other compounds. The following are a few examples of these applications: Ethyne is most commonly used to make organic compounds such as ethanol, ethanoic acid, and acrylic acid.
Tests for the Presence of a Triple Bond
The presence of a triple bond in any hydrocarbon makes it unsaturated and the compound gives Baeyer’s test. However, alkynes are characterised by certain specific tests described below.
- With ammoniacal silver nitrate: Alkynes give a white precipitate of silver acetylide with ammoniacal silver nitrate.
- With ammoniacal cuprous chloride: Alkynes give a red precipitate of cuprous acetylide with ammoniacal cuprous chloride.
Uses of Alkyne
- Since ethyne has a very hot flame, it is commonly used in oxyacetylene gas welding and oxyacetylene gas cutting. As ethyne is burned with oxygen, the resulting flame is known to have a temperature of about 3600 Kelvin.
- The overriding alkyne in acetylene is used as a fuel, with millions of kilograms created annually by fractional oxidation of natural gases. Chemical compounds such as ethanoic acid, acrylic acid, and ethanol are made from some of these alkynes.
- Ethyne is most commonly used to make organic compounds such as ethanol, ethanoic acid, and acrylic acid. It’s also used to make polymers and the raw materials for them.
- Acetylene is broken down into its two components, carbon and hydrogen. This reaction creates a lot of heat, which can cause the gas to ignite even if there is no air or oxygen present.
- Alkynes are generally used as the starting materials for the manufacture of a large number of organic compounds of industrial importance such as chloroprene, vinyl chloride etc.
Frequently Asked Questions – FAQs
What is the structural formula of Ethyne?
The formula for ethyne condensed structure is HC≡CH. C2H2 is the molecular formula for ethyne. That means ethyne has two carbon atoms and two hydrogen atoms, with the two carbon atoms bound together by triple bonds.
How are alkynes named?
Higher alkenes and alkynes are called by adding a -ene (alkene) or -yne (alkyne) suffix to the stem name of the unbranched alkane with that number of carbons and counting the number of carbons in the longest continuous chain that includes the double or triple bond.
What is an alkyne group?
Alkynes have the empirical formula CnH2n-2 and are organic molecules with the functional group carbon-carbon triple bonds. They are hydrocarbons that are unsaturated. Alkynes have the suffix –yne, which is used when there is just one alkyne in the molecule, similar to how alkenes have the suffix –ene.
How are alkynes prepared?
To make a vicinal dihalide from an alkene, chlorine or bromine is combined with an inert halogenated solvent like chloromethane. The formed vicinal dihalide is then heated and reacted with a strong base to create an alkyne.
How can we reduce alkyne to alkene?
With sodium dissolved in an ammonia solution, alkynes can be converted to trans-alkenes. In a carbon-carbon triple bond, a Na radical donates an electron to one of the pi bonds. In an ammonia solution, this creates an anion, which can be protonated by hydrogen.

Comments