What is Non-Metal?
Non-metals are the elements which form negative ions by accepting or gaining electrons. Non-metals usually have 4, 5, 6 or 7 electrons in their outermost shell.
Table of Contents
- Properties of Non-metals
- Physical Properties of Non-Metals
- List of Non-Metals
- Chemical Properties of Non-Metals
- Recommended Videos
- Uses of Non-Metals
- Frequently Asked Questions – FAQs
Non-metals are those which lack all the metallic attributes. They are good insulators of heat and electricity. They are mostly gases and sometimes liquid. Some of then are even solid at room temperature like Carbon, sulphur and phosphorus.
Properties of Non metals
Characteristic properties of non-metals are high ionization energies and high electronegativity. Owing to these properties, non-metals usually gain electrons when react with other compounds, forming covalent bonds.
The following are the general properties of non-metals.
- The atoms of non-metals tend to be smaller than those of metals. Several of the other properties of non-metals result from their atomic sizes.
- Non-metals have high electronegativities. This means that the atoms of non-metals have a strong tendency to attract more electrons than what they would normally have.
- Non-metals have high electronegativities. This means that the atoms of non-metals have a strong tendency to hold on to the electrons that already have. In contrast, metals rather easily give up one or more electrons to non-metals, metal therefore easily form positively charged ions, and metals readily conduct electricity.
Physical Properties of Non-Metals
- Under normal conditions of temperature and pressure, some non-metals are found as gases, some found as solids and one is found as liquid. In contrast, except mercury, all metals are solids at room temperature. The fact that so many non-metals exist as liquids or gases means that non-metals generally have relatively low melting and boiling points under normal atmospheric conditions.
- In their solid-state, non-metals tend to be brittle. Therefore, they lack the malleability and ductility exhibited by metals.
- Ductility is the property of the material to be stretched into wires but non-metals are not ductile except for carbon, as carbon fibres find uses in a wide variety of industries including sports and music equipment.
- Another property characteristic to metals is absent in non-metals called malleability. They can’t be drawn into sheets as they are brittle and break on applying pressure.
- Non-metals exhibit very low electrical conductivities. The low or non-existent electrical conductivity is the most important property that distinguishes non-metals from metals.
- They are not sonorous and do not produce a deep ringing sound when they are hit with another material. They are also bad conductors of heat and electricity except for graphite.
List of Non-Metals (the Complete List)
| Non-metal | State at Room Temperature | Symbol |
| Hydrogen | Gas | H |
| Nitrogen | Gas | N |
| Oxygen | Gas | O |
| Fluorine | Gas | F |
| Chlorine | Gas | Cl |
| Bromine | Liquid | Br |
| Iodine | Solid | I |
| Carbon | Solid | C |
| Sulphur | Solid | S |
| Phosphorous | Solid | P |
| Silicon | Solid | Si |
Chemical Properties of Non-Metals
1. Reaction with Water
A non-metal does not react with water but it is usually very reactive in air, which is why some of them are stored in water. For example, one of the highly reactive non-metals is phosphorus and it catches fire when exposed to air that is why it is stored in water to prevent its contact with atmospheric oxygen.
2. Reaction with Acids
None of the non-metals is known to react with acids.
3. Reaction with Bases
The reaction between non-metals and bases is a very complex one. The reaction of chlorine with bases like sodium hydroxide gives products like sodium hypochlorite, sodium chloride as well as water.
4. Reaction with Oxygen
Oxides of non-metals are formed when it reacts with oxygen. The oxides of non-metals are acidic or neutral in nature.
When sulphur reacts with oxygen, we get sulphur dioxide.
S + O2 → SO2
When sulphur dioxide reacts with water it forms sulphurous acid.
SO2 + H2O → H2SO3
5. Reaction with metal
Non-Metals react with metal, generally forming Ionic compounds.
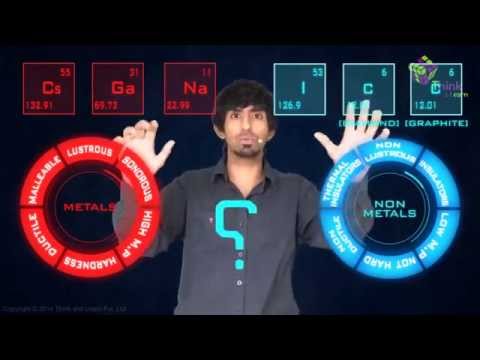
Recommended Videos
Physical Properties of Metals and Non-metals
Uses of Non-Metals
- For the preparation of ammonia, nitric acid and fertilizers, nitrogen is used.
- For the purification of water, chlorine is used.
- Hydrogen is very useful as rocket fuel.
- Carbon can be used to make pencils when it is in the graphite form.
- Sulphuric acid is prepared using sulphur.
Frequently Asked Questions – FAQs
What are examples of non-metals?
Hydrogen, chlorine, fluorine, carbon, nitrogen, phosphorus, selenium are examples of non-metal.
What defines non-metal?
A chemical element (such as boron, carbon or nitrogen) that lacks metal properties and is capable of forming anions, acid oxides, acids, and stable hydrogen compounds.
What are non-metal and metal?
Non-metals are such elements which have 4,5, 6 and 7 electrons in their outermost shell. Examples of non-metals are carbon, oxygen chlorine etc.
Metals are such element which have generally 1,2,3 valence electrons. Example of some metals are Sodium, Potassium, Copper etc.
What are non-metallic materials?
Non-metals are natural materials that do not produce heat or electricity and that are structurally brittle (can not be easily rolling, moulding, extruding or pressing). Hydrogen, carbon, nitrogen, oxygen, phosphorus, arsenic and selenium are the non-metallic elements in the periodic table.
What is called ductility?
Ductility is a measure of the ability of a metal to withstand tensile stress — any force that separates the two ends of an object from each other. Literally, the word “ductile” means a metal material can be stretched into a thin wire without being weaker or more fragile in the process.
Is plastic non-metal?
For elements, the term metal and non-metals are used. Plastic is not an element but a polymer consisting of various non-metals such as carbon, hydrogen, oxygen, nitrogen and so on. Not all plastic can be deformed, either.
What is a liquid metal called?
Liquid metal is composed of alloys with very low melting points that form a liquid eutectic at room temperature. The main metal used to be mercury, but in different applications, gallium-based alloys, which are lower in both their vapour pressure at room temperature and toxicity, are used as a replacement.
For more information contact BYJU’S mentors.


Why water is the most universal and commonest solvent in the univers?
Approximately 71% of the Earth’s surface is covered by water. This compound has the ability to dissolve a wide spectrum of substances. This is the reason why water is called a universal solvent.
Am highly greatful in the knowledge been gain in these content
why is hydrogen the most abundant gas in space??
Hydrogen is the simplest element since it contains only one proton and one electron. This is believed to be the reason behind its abundance in the universe.
What is fractional atomic mass
Most elements’ atomic masses are fractional, since they exist as a combination of various mass isotopes. Many of the elements exist as a combination of the varying mass isotopes. Out of this combination the fractional atomic masses emerge. Avg Avg. Mass = total mass of all atoms / atomic number.
Thanks you sir for help me। 😀😀😀
Write the properties of non-metals:phosphorus?
Phosphorus (P), nonmetallic chemical element of the nitrogen family (Group 15 [Va] of the periodic table) that at room temperature is a colourless, semitransparent, soft, waxy solid that glows in the dark.