Table of Content
Organic Chemistry Some basic principles and TechniquesGeneral IntroductionTetravalence of Carbon Shapes of Organic CompoundsStructural Representations of Organic CompoundsClassification of Organic CompoundsNomenclature of Organic CompoundsIsomerismFundamental Concepts in Organic Reaction MechanismMethods of Purification of Organic CompoundsQuantitative Analysis
Organic chemistry began to emerge as a science about 200 years ago. By the late eighteenth century, substances were divided into two classes called organic and inorganic compounds. Organic compounds were obtained from plants and animals. Organic compounds were more difficult to work within the laboratory and decompose easily.
What exactly makes a molecule organic?
The answer is it is a single precious atom – CARBON. All organic molecules contain carbon, and to study organic chemistry is to study molecules made of carbon and to see what kinds of reactions they undergo and how they are put together. Therefore, Organic chemistry is the branch of chemistry that deals with the study of carbon compounds.
Organic Chemistry Class 11 Chapters
The chapter-wise list covered in NCERT Organic Chemistry Class 11 is tabulated below.
| Section Number | Chapter Name |
| 12 | Organic Chemistry – Some basic principles and Techniques |
| 12.1 | General Introduction |
| 12.2 | Tetravalency of Carbon (Shapes of Organic Compounds) |
| 12.3 | Structural representations of Organic Compounds |
| 12.4 | Classification of Organic Compounds |
| 12.5 | Nomenclature of Organic Compounds |
| 12.6 | Isomerism |
| 12.7 | Fundamental Concepts in organic Reaction Mechanism |
| 12.8 | Methods of Purification of Organic Compounds |
| 12.9 | Quantitative Analysis |
Recommended Videos
Previous Year Paper Solutions – NEET

Identify the Types of Organic Reactions

IUPAC Names of Organic Compounds

Meaning of GOC in Chemistry

Organic Chemistry-Top 7 Questions

Organic Chemistry-Top 5 Questions

Organic Chemistry Class 11 NCERT
Organic Chemistry Some basic principles and Techniques
- Organic Chemistry – Organic chemistry is the study of carbon compounds that always contain carbon and it is limited to other elements. Compounds obtained from plants and animals were termed organic to indicate their ultimate source was a living organism.
Also, Read: Important Question on Organic Chemistry
General Introduction to Organic Chemistry
Organic chemistry is one of the most important disciplines of science which deals with the study of carbon compounds especially hydrocarbons and their derivatives.
Also, Read: Introduction to Organic Chemistry
Tetravalency of Carbon Shapes of Organic Compounds
- Catenation – Catenation can be defined as the self-linking of atoms of an element to form chains and rings. This definition can be extended to include the formation of layers (two-dimensional catenation) and space lattices (three-dimensional catenation).
- Tetravalency and small size – Carbon exhibits’ tetravalency. The tetravalency of carbon can be satisfied by forming bonds with carbon, hydrogen or other atoms. The carbon atom has 4 electrons in its valence shell. In order to account tetravalency it is believed during the process of bond formation which is energy-releasing process the two electrons in the 2s orbital get unpaired and out of them, one is promoted to empty orbital.

Also Read: Tetravalency of Carbon
Structural Representations of Organic Compounds
- Complete Structural Formula – Full structural equations show all the atoms in a molecule, the types of bonds that bind them, and how they are interconnected.
- Condensed Structural Formula – The Condensed structural formula is used to save space, structural formulas are conveniently abbreviated as condensed structural formulas.
- Bond Line Structural Formula – A bond-line structure is a less cluttered drawing than a condensed structural formula. However, to understand the simplified bond-line structure, the reader has to mentally add many more features to comprehend the overall structure.
Also Read: Structural Formula
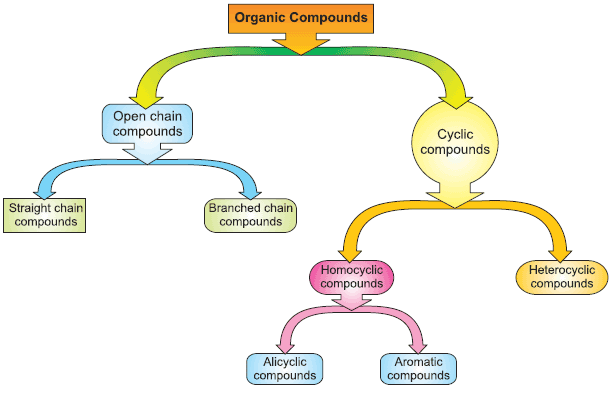
Classification of Organic Compounds
- Acyclic or Open Chain Compounds & Alicyclic or Closed Chain or Ring Compounds – Organic compounds are classified as open-chain compounds and closed chain compounds in terms of the carbon chain. Also termed as Organic Compounds Acyclic or Open Chain or Aliphatic Compounds, Cyclic or Closed Chain or Ring Compounds.
- Aromatic Compounds – Plants and micro-organisms have an exclusive route to benzene-ring compounds. The great majority of aromatic compounds in nature, therefore, are produced by plants and microorganisms, and animals are dependent upon plants for many aromatic compounds either directly or Indirectly.
- Heterocyclic Aromatic Compounds – In the twentieth century it is witnessed that the first inorganic heteroaromatic compound produced in the laboratory. Some of these heterocyclic aromatic compounds are very important in biochemical processes, drugs, and agrochemicals.
Also, Read: Aromatic Compounds
Nomenclature of Organic Compounds
- IUPAC Rules – Nomenclature of Organic Compounds follows recommendations of IUPAC in naming organic compounds, carbocations, etc. The International Union of Pure and Applied Chemistry (IUPAC) formulated rules for naming organic compounds.
- Types of Chemical Nomenclature – Chemical nomenclature is based primarily on naming a presumed geometrical arrangement of atoms. The International Union of Pure and Applied Chemistry(IUPAC) maintains several commissions that deal with the naming of chemical substances. In general, the approach of IUPAC is to present rules for arriving at names systematically.
Also, Read: IUPAC Nomenclature
Isomerism
- Structural Isomerism – Structural Isomerism arises due to different arrangement of atoms within the molecule. Two molecules are structural isomers if they share the same molecular formula.
- Stereoisomerism – Stereo-isomers are isomeric molecules having the same molecular formula and the same sequence of bonded atoms, but are only different in the 3D orientations of their atoms in space. Stereoisomerism may be of two types viz. geometrical (or cis-trans) isomerism and optical (or d-l or mirror-image) isomerism.
Also, Read: Isomerism
Fundamental Concepts in Organic Reaction Mechanism
- The Shapes of Carbon Compounds – Tetravalent carbon atom is the building block of structural organic chemistry. The four hydrogen atoms, with four carbon atoms, form a structure known as a tetrahedron.
- Functional Groups – Functional groups were introduced as a useful method for organizing this vast number of compounds because chemical reactions occur at the functional group and compounds with the same functional group undergo similar reactions.
- Homologous Series – A series of organic compounds in which every succeeding member differs from the previous one called Homologous Series.
Also, Read: Functional Groups
Methods of Purification of Organic Compounds
- Simple crystallization – Crystallization is one of the most effective purification techniques for solids. Simple crystallization involves the selection of the solvent and preparation of the solution.
- Fractional crystallization – Fractional crystallization is used for the purification of a single substance contaminated with small quantities of impurities.
- Sublimation – Sublimation is an excellent method for purifying relatively volatile organic solids on scales ranging from a few milligrams to tens of grams.
- Simple distillation – Simple distillation is the process of converting a liquid into its vapour, transferring the vapour to another place, and recovering the liquid by condensing the vapour.
- Fractional distillation – Fractional distillation is the separation procedure of a mixture into sections or fractions of its material. By heating them to a temperature at which one or more parts of the mixture vaporize, chemical compounds are isolated.
- Steam distillation – A steam distillation is simply a distillation in which steam is involved as a process component. Steam distillation and organic solvent extraction have both been widely used to extract compounds from spices.
- Azeotropic distillation – Azeotropic distillation is accomplished by adding to the liquid phase, a volatile third component that changes the volatility of one of the two components more than the other so that the components are separated by distillation.
- Chromatography – The purpose of preparative chromatography is to separate the components of a mixture for further use (and is thus a form of purification).
Also, Read: Purification of Organic Compounds
Quantitative Analysis
The elementary organic analysis is also considered, along with the development of the theory of analytical chemistry. Quantitative analysis refers to assessing how much is present in a sample of a given item. The quantity of any or all components of a sample can be expressed in terms of size, concentration, or relative abundance.
Also, Read: Salt Analysis
Frequently Asked Questions – FAQs
What is meant by organic compounds?
Organic compound, one of a large class of chemical compounds in which one or more carbon atoms are covalently paired with other elements, atoms, most commonly hydrogen, oxygen, or nitrogen.
What is an example of an organic chemical?
Types include gasoline, plastics, detergents, colorants, food additives, natural gas, and drugs. Soap and detergent are two different examples of organic chemistry, although both are used for washing.
What are the uses of organic compounds?
Organic molecules are used in a variety of industries in human society, including food, pharmaceuticals, fuels, and building, to name but a few. Alkanes include chemical substances such as propane, octane, and methane. These are commonly used as oils for items like gasoline in the car and heating/cooking oil in the home.
Why are organic compounds useful?
Organic compounds are essential because they contain carbon in all living organisms. They are the basic components that move the world in many of the cycles. For example, the carbon cycle which involves exchanging carbon in photosynthesis and cell respiration between plants and animals.
What organic compounds are used in medicine?
Compounds used as medicinal products are most usually organic compounds, sometimes divided into large groups of small organic molecules (e.g., atorvastatin, fluticasone, clopidogrel) and “biologics” (infliximab, erythropoietin, insulin glargine), the latter more widely used as protein medicinal products.

Nice very effective teaching which helps to seek some knowledge about chemistry which is important