The azo products obtained when diazonium salts react with aromatic compounds have an extended conjugated system through N = N bond. This reaction is called a coupling reaction and the products formed are coloured.
2-Naphthol aniline dye is a red dye with an azo compound. It is primarily used for textile dyeing. 2-Aniline naphthol dye is made from aniline.
Aim:
To prepare 2-Naphthol Aniline Dye from aniline, sodium nitrite, hydrochloric acid and alkaline solution of β-napthol also called 2-naphthol.
Theory:
2- Naphthol aniline dye is a scarlet dye that can be prepared by coupling reaction. Aniline reacts with sodium nitrite in the presence of hydrochloric acid to form benzene diazonium chloride. Further benzene diazonium chloride reacts with 2-naphthol forms a bright orange colour 2-naphthol and forms aniline dye.
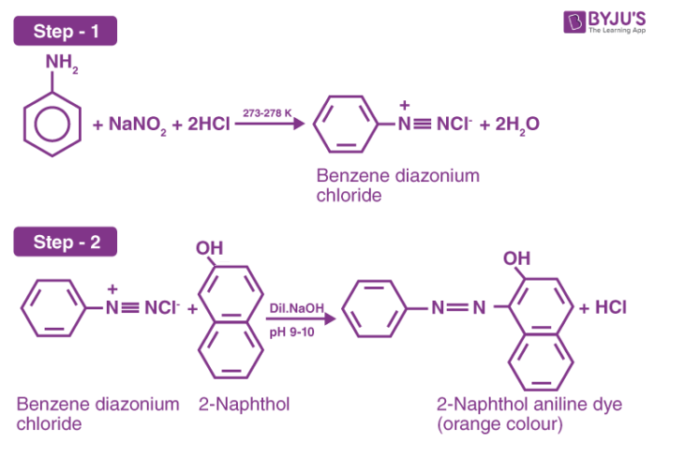
Azo compounds are prepared by the reaction of diazonium salts with phenol under alkaline conditions. Primary aromatic amines react with nitrous acid at 0 oC to give a diazonium salt. Nitrous acid is in turn formed by the reaction of sodium nitrite with hydrochloric acid. The active reagent is nitrous anhydride or dinitrogen trioxide. Nitrous anhydride reacts with aniline to give a nitroamine derivative which is unstable and isomerizes to form a diacetic acid which in turn is converted to a diazonium salt. Finally, this diazonium salt reacts with 2-naphthol in the presence of sodium hydroxide to give 2-naphthol aniline which is an aniline dye.
| Also Read: Preparation of 2-Naphthol Aniline Dye Viva Questions |
Materials Required:
- Aniline
- Sodium nitrite
- Hydrochloric acid
- 2-Naphthol
- Sodium hydroxide solution
- Beaker
- Test tube
- Buchner funnel
- Pipette
- Capillary tube
- Thermometer
- Distilled water
- Hot air oven
- Ice bath
Apparatus Setup:
Procedure:
- Dissolve 5 ml of aniline in a mixture of concentrated hydrochloric acid and water.
- Cool the solution in an ice bath between 0-5 oC.
- Add a solution of 4 gm sodium nitrite in 15 ml of water dropwise with continuous shaking and controlling the temperature below 5 oC.
- Take another flask to dissolve 8 gm of 2-naphthol in a solution of 5 gm sodium hydroxide solution in 50ml of water.
- Cool the solution in the ice bath to 0-5oC.
- Now mix the two cold solutions slowly dropwise with constant stirring.
- Continue the stirring for at least half an hour without allowing the temperature to rise above 10oC.
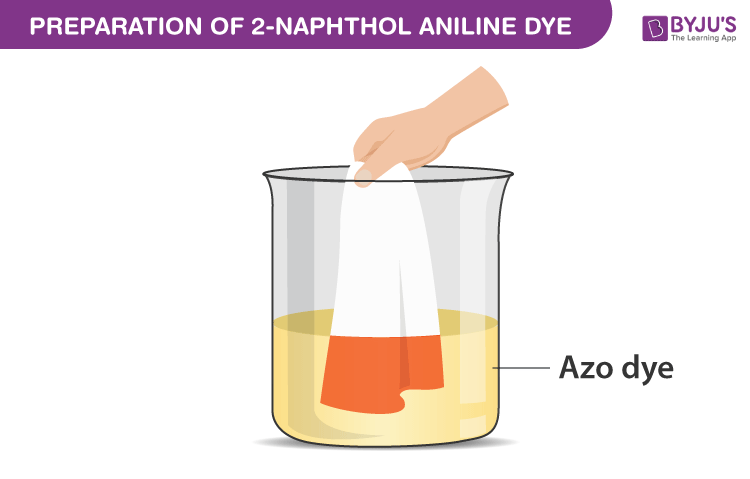
- An orange colour azo dye called 2-naphthol aniline separates out.
- Filter the crude sample and wash it with cold water.
- Dry and recrystallise it from ethyl alcohol or glacial acetic acid.
Observations:
| Colour of the crystals | Orange crystals |
| Expected Yield | 13 gm |
| Melting Point | 131 oC |
Results and Discussion:
The yield of 2- Naphthol aniline dye is ______ gm.
Precautions:
- The solution must be cooled to 5 oC. Do not raise the temperature.
- Do not touch the dye otherwise it will stick to hands.
- Not to touch the concentrated acids otherwise, it will cause irritation.
- Wash the crude sample repeatedly with cold water in order to remove soluble impurities.
- Maintain the pH between 4-5.
Keep visiting BYJU’S to learn more about class 12 CBSE chemistry practicals.
Frequently Asked Questions on Preparation of 2-Naphthol Aniline Dye
What are the applications of azo dye?
Azo dyes are predominantly used in textile, paper manufacturing and printing units. They work as an artificial colourant.
Give the formula of 2-naphthol aniline dye.
The formula for 2-naphthol aniline is C16H12N2O and it is a scarlet red dye.
What is a diazotization reaction?
Diazotization reaction is the reaction of primary aromatic amines with nitrous acid to form diazonium salt. Usually, this reaction is carried out at a very low temperature.
What is the colour of pure aniline?
Pure aniline is star yellow in colour but turns red-brown on exposure to air and light.


Comments