What are Noble Gases or Inert Gases?
The elements in Group 18 of the periodic table are known as the noble gases. These elements are also known as the inert gases, which is a very appropriate name for them since this group of elements exhibits extremely inert chemical behaviour.
Table of Content
- Members of the Noble Gas Family
- Recommended Videos of Noble Gases
- Non-Metallic Behaviour of Inert Gases
- Uses of Noble Gases
- How were the Inert Gases Discovered?
- FAQs
Members of the Inert Gas Family
The members of group 18 in the modern periodic table are:
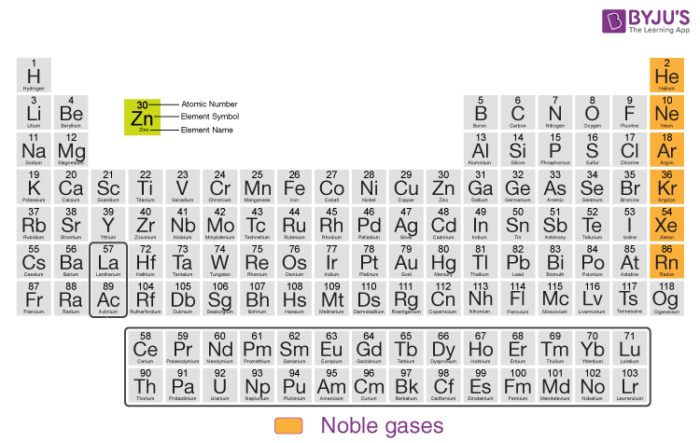
These gases are so-called due to the stability of these elements. This stability is due to the completely filled outermost shells of the elements. The inert gases are used in various applications irrespective of their inert or scarcely reactive nature.
Related Videos on Noble Gases
Non-Metallic Behaviour of Inert Gases
The inert gases are typical non-metals in many ways. They are colourless, odourless gases which have low melting and boiling points. They are poor conductors of heat and electricity even when in the liquid form. Just like in other groups of the periodic table, there are clear trends in the melting and boiling points of the inert gases. The melting and boiling points increase down the group – although as the boiling points increase down the group – although as the boiling point of radon is still only -62oC they all boil at pretty low temperatures.
The density of the inert gases also increases going down the group, the result of the atoms getting bigger all the time. Helium has the smallest and lightest atoms and so helium gas is less dense than air, a fact which is important when we look at it.
Uses of Noble Gases
- In metallurgical processes, argon is widely used in order to provide the necessary inert atmosphere. This inert atmosphere plays an important role in welding titanium, aluminium, stainless steel, and magnesium. It is also used in the production of titanium.
- A limited amount of argon is used in germanium and silicon crystals which are used in electric light bulbs, transistors, etc.
- The boiling point of helium is the least when compared to any other liquid. It is used to obtain the lowest temperatures required in lasers.
- Helium is used in nuclear reactors as a cooling gas and used as a flow-gas in liquid-gas chromatography. It finds its application in airships and helium balloons.
- Helium balloons are used to check the weather of a particular region. Helium is preferred over hydrogen though hydrogen is cheaper, as helium is readily available and hydrogen is highly inflammable. Hence, due to safety issues helium is preferred in aircraft.
- It is used by divers to dilute oxygen over nitrogen in the gas cylinders used by them as nitrogen can easily be dissolved in the blood which results in a painful condition called bends. The risk of helium causing bends is slightly lower than nitrogen.
- Neon is used in discharge tubes which is the reason behind the reddish-orange glow produced by neon lights.
- Xenon and krypton find their application in photographic flash units due to the generation of very bright light. It is also used in lighthouses.
- Neon, xenon, and krypton are used to produce different colour lights.
How were the Inert Gases Discovered?
The inert gases are invisible and unreactive and this makes them very difficult to spot when they are mixed with other gases in air. In 1894, there was no reason to suspect they existed at all.
In that year Sir William Ramsay did an interesting experiment. He wanted to see what happened when all the gases were removed from the air. He did this by passing air over heated copper and heated magnesium. He expects to have nothing left at the end. He reasoned that everything in the air would react, either with the hot copper or with the hot magnesium. In fact, he found that from every 100cm3 of air about 1cm3 always remained behind.
William Ramsay was such a good scientist that he knew he hadn’t made an error. No matter how often he repeated the experiment, he always had the same proportion of gas left behind. He did lots of experiments on the leftover gas to try and make it react with something. He tried the most reactive substance he knew, including fluorine, phosphorus and potassium, but it never did anything. So he called this rather boring new gas argon, from a Greek word meaning lazy or inactive.
Frequently Asked Questions – FAQs
Why are inert gases important?
The noble gases often do not react with many substances and have been historically called inert gases. Generally, inert gases are used to prevent unwanted chemical reactions which degrade a sample. Oxidation and hydrolysis reactions with the oxygen and moisture in air are often these undesirable chemical reactions.
Why inert gases do not react?
These atoms’ full-valence electron shells make noble gases highly stable and difficult to form chemical bonds, since they continue to gain or lose electrons. Although noble gasses usually do not react to form compounds with other elements, there are some exceptions.
What are the properties of the noble gases?
Other characteristics of the noble gasses are that they all conduct electricity, fluorescent, are odourless and colourless, and are used under several conditions where a stable element is needed to maintain a healthy and constant environment. This series of chemicals contains helium, neon, argon, krypton, xenon and radon.
Why are noble gases called noble?
The noble gases are helium, argon, krypton, xenon, and radon, in order of their mass. They are called noble gases because they are so majestic that they do not react with anything in general. They’re also known as inert gases for this reason.

This really helped to gain knowledge about Noble gas.
But can you tell why the exeption like Xenon use to make bond with Florine and Oxygen?
The noble gas elements do not react with other elements, as they are less electronegative and unable to attract the lone pair of electrons from noble elements. Some examples of noble gas compounds are XeF2, XeF4, XeF6, XeO3, XeOF2 etc. Due to this, the noble gases form compounds with fluorine and oxygen only.