
| Symbol | Ar |
| Atomic Number | 18 |
| Atomic Mass | 39.948 g.mol-1 |
| Discovered by | Sir Ramsay in 1894 |
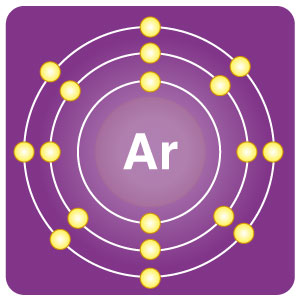
Table of Contents
- What is Argon?
- Properties Of Argon
- Chemical Properties Of Argon
- Uses Of Argon
- Certain Facts About Argon
- Frequently Asked Questions – FAQs
What is Argon?
Argon is a chemical element in the eighteen group of the periodic table. It is a noble gas, and it is the third most abundant gas in earth’s atmosphere.
Argon is the most common gas in the atmosphere besides Nitrogen and Oxygen. Argon is a noble gas (like helium) which means that it is completely inert.
Properties Of Argon
- It is odourless, colourless gas that is totally inert into other substance.
- Under extreme conditions, argon can form certain compounds even though it is a gas.
- It is characterized by same solubility level in water as that of oxygen.
- It has low thermal conductivity.
Chemical Properties Of Argon
| Group | 18 | Melting point | -189 oc |
| Period | 3 | Boiling point | -185.7 oc |
| Block | p | Density (g cm−3) | 0.001633 |
| Atomic number | 18 | Relative atomic mass | 39.948 |
| State at 20°C | Gas | Key isotopes | 40Ar |
| Electron configuration | [Ne] 3s23p6 | CAS number | 7440-37-1 |
| ChemSpider ID | 22407 | ChemSpider is a free chemical structure database | |
Uses Of Argon
- They are used in metal industries.
- It is used in the production of titanium.
- It is used in double dazzled windows to fill the space between the panels.
Certain Facts About Argon
- Argon was suspected to be present in air by Henry Cavendish in the year 1785.
- According to Chimcool, the majority of argon is the isotope argon-40 which emerge from radioactive decay of potassium-40.
Recommended Videos
The Modern Periodic Table

Frequently Asked Questions – FAQs
What is argon used for?
When an inert atmosphere is required, argon is frequently employed. It is employed in the synthesis of titanium and other reactive elements in this way. Welders use it to shield the weld area, and incandescent light bulbs use it to keep the filament from corroding due to oxygen.
Why is argon purple?
The argon atom is made up of 18 protons and 18 electrons. It has eight electrons in its outer shell. Argon is a colorless and odorless gas under normal conditions. When argon is activated by a high-voltage electric field, it emits a violet-colored light.
What happens if you inhale argon gas?
This gas is categorized as a basic asphyxiant because it is inert. Excessive concentrations can cause dizziness, nausea, vomiting, loss of consciousness, and death when inhaled. Errors in judgement, confusion, or loss of consciousness can all lead to death if self-rescue is not possible.
What group is argon in?
Gases that are noble or inert. Helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Ro) are the noble gases or inert gases in Group 8A (or VIIIA) of the periodic table (Rn). The name derives from the fact that certain elements are almost non-reactive with other elements or compounds.
Is argon used in medicine?
Argon has also been used as a surgical instrument in the medical field. Argon Plasma Coagulation (APC) is a non-contact technique that uses high-frequency argon plasma stimulation to cauterise surrounding tissues and control bleeding around surgical sites through coagulation.


Comments