Methanol (CH3OH) and ethanol (C2H5OH) are the simplest members of the primary alcohol family and have a wide range of applications in the fuel industry. Some important uses of methanol and ethanol are listed in this article.
Table of Contents
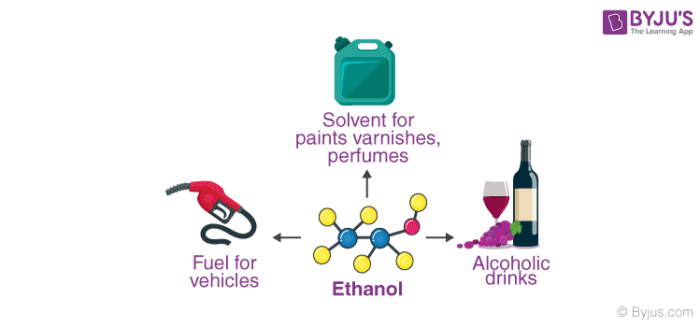
Uses of Ethanol
- Owing to its antibacterial and antifungal properties, ethanol (also known as ethyl alcohol) is used in many hand sanitizers and medical wipes.
- Ethanol is also used as an antiseptic and as a disinfectant.
- In cases of ethylene glycol poisoning or methyl alcohol poisoning, ethanol is often administered as an antidote.
- Several medications that are insoluble in water are often dissolved in ethanol. For example, ethanol (in concentrations ranging from 1% to 25%) is used as a solvent for some analgesics and mouthwashes.
- Ethanol is the primary ingredient in many alcoholic drinks that are orally consumed for recreational purposes. It acts as a psychoactive drug by reducing anxiety and creating a feeling of euphoria in Humans. However, it also impairs cognitive and motor functions and acts as a central nervous system (CNS) depressant.
- Ethanol is used industrially in the production of ethyl esters, acetic acid, diethyl ether, and ethyl amines.
- This compound is widely used as a solvent due to its ability to dissolve both polar and nonpolar compounds.
- Since it has a melting point of -114.1oC, ethanol is used as an ingredient in cooling baths in several laboratories. It also serves as the active fluid in many spirit thermometers.
Click here to learn more about ethanol.
Uses of Ethanol as a Fuel
Ethanol is widely used as a fuel additive and as an engine fuel. Some forms of gasoline are known to contain up to 25% ethanol. This compound has also been used as rocket fuel in some bipropellant rockets. When used in fuel, ethanol is believed to reduce carbon monoxide and nitrogen oxide emissions.
Since it is widely available and has low toxicity and cost, ethanol is used in direct-ethanol fuel cells (or DEFCs). However, commercially used fuel cells generally use methanol, hydrogen, or natural gas.
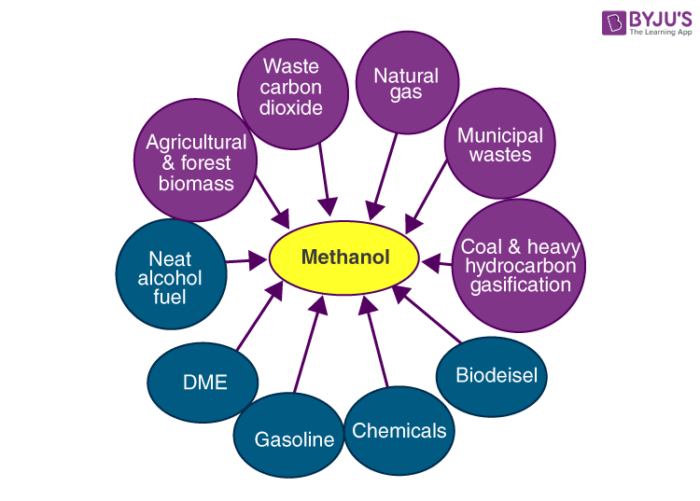
Uses of Methanol
- Methanol is widely used in the production of acetic acid and formaldehyde.
- In order to discourage the recreational consumption of ethanol, methanol is often added to it as a denaturant.
- This compound is also used as an antifreeze (an additive that is used to lower the freezing point of a liquid) in many pipelines.
- It is also used in sewage treatment plants since it serves as a carbon-based food source for denitrifying bacteria.
- The polyacrylamide gel electrophoresis (PAGE) technique involves the use of methanol as a destaining agent.
- A mixture of water and methanol is used in high-performance engines in order to increase power.
- Methanol is used in the production of hydrocarbons, olefins, and some aromatic compounds.
- It is also used in the production of methyl esters and methylamines.
Click here to learn more about methanol.
Uses of Methanol in Fuel
Methanol can be used as a fuel in several internal combustion engines. The chemical equation for the burning of methanol is given by:
2CH3OH + 3O2 → 4H2O + 2CO2
However, the primary disadvantage of methanol as a fuel is that it has a tendency to corrode aluminium and some other metals. Another shortcoming of methanol as a fuel is that its energy density is approximately half of the energy density offered by gasoline.
An advantage of methanol as a fuel is that it is relatively easy to store. The storage of liquid methanol is much easier than the storage of hydrogen gas or natural gas. Other merits of this compound include its biodegradability and its short half-life in groundwater.
Frequently Asked Questions – FAQs
What are the main uses of methanol?
Methanol is a highly versatile chemical, also known as methyl alcohol, commonly used for manufacturing applications and ubiquitous in our daily lives. As an energy carrier, its performance has made it increasingly popular as a fuel for factories and for producing electricity.
What is the difference between methanol and ethanol?
The molecular structure of two carbon atoms breaks into ethanol, which is also known as ethyl alcohol. Methanol, also known as methyl alcohol, consists of only one atom of carbon.
What are the two uses of ethanol?
It is used as a solvent, in the manufacture of other organic compounds, and as an additive to automobile fuel (a mixture known as gasohol). Ethanol is a major industrial chemical. The intoxicating portion in many alcoholic drinks, such as beer, wine, and distilled spirits, is ethanol.
What is the molecular formula for methanol and ethanol?
The molecular formula for methanol and ethanol is CH3OH and C2H5OH respectively.
To learn more about the uses of methanol, ethanol, and other important alcohols such as benzyl alcohol, register with BYJU’S and download the mobile application on your smartphone.

Is methanol good antiseptic and germs cleaner then ethanol?
While both of these alcohols (methanol and ethanol) are known for their antiseptic qualities, certain studies suggest that ethanol is the better antiseptic.
Can we use methanol or wood alcohol to make hand sanitizer?
No, methanol should not be used in hand sanitizers or even surface disinfectants. This is because methanol can cause serious damage to internal organs if ingested or absorbed through the skin. Safe alternatives that can be used in hand-sanitizers include ethanol and isopropanol.