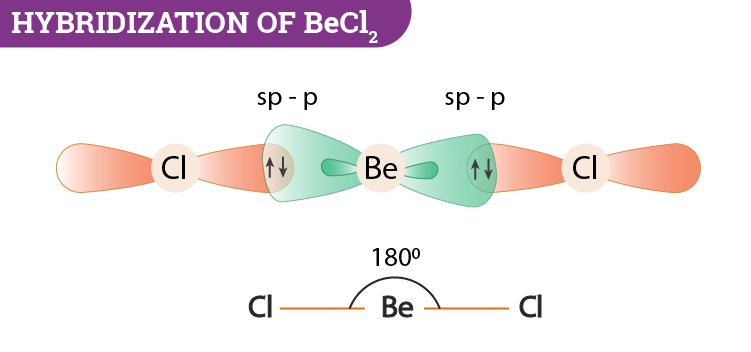
There are different types of hybridization that usually occur in BeCl2. However, beryllium dichloride in the gaseous state exists in a linear form and is sp hybridized.
| Name of the Molecule | Beryllium Dichloride |
| Molecular Formula | BeCl2 |
| Hybridization Type | sp |
| Bond Angle | 1800 |
| Geometry | Linear |
What is the Hybridization of Beryllium Dichloride?
To know about the hybridization of BeCl2 (Beryllium Dichloride) we have to take a closer look at the central atom which is Be. Its electronic configuration is 1s2, 2s2, where two electrons are present in the valence shell. During the formation of BeCl2, beryllium atom bonds with two chlorine atoms via single covalent bonds. The number of electron pairs around the central atom will be two. No lone pair is found in the molecule. If we analyse this information then we can conclude that BeCl2 has sp hybridization.
Important Points To Remember
- In the BeCl2 molecule, beryllium dichloride forms single covalent bonds with two chlorine atoms each.
- The central atom Be will consist of two bond pairs.
- No lone pair exists.
BeCl2Molecular Geometry And Bond Angles
BeCl2 molecular geometry is said to be a linear one with a bond angle of 180o. It is a non-polar molecule because they have less attraction between each other.
Read More About Hybridization of Other Chemical Compounds
- Hybridization Of BF3
- Hybridization Of I3
- Hybridization Of XeF2
- Hybridization Of ClF3
- Hybridization Of Ethene

Comments