Functional Groups, in the field of organic chemistry, are the substituent atoms or groups of atoms that are attached to specific molecules. These moieties (the part of the molecule which can be found in many other molecules as well) are responsible for the chemical reactions that the molecule they are attached to participate in.
A functional group is a group of atoms or bonds inside a substance that is responsible for the substance’s unique chemical reactions in organic chemistry. Regardless of the chemical in which it is found, the same functional group will behave similarly and experience comparable reactions.
Table of Contents
- What are Functional Groups?
- Recommended Videos
- Role of Functional Groups
- Nomenclature of Common Functional Groups
What are Functional Groups?
Functional Groups are a “particular grouping of components in which the distinctive chemical reactions of these molecules are accountable”.
Two molecules having different sizes but the same functional groups will take part in chemical reactions that are similar or exactly the same. The presence of a functional group in a molecule implies that the behaviour and the chemical reactions of the molecule in question can be predicted in a systematic fashion.
The process of chemical synthesis, in which chemical reactions are intentionally executed in order to obtain a specific compound, can be designed by understanding the properties of various functional groups.
Recommended Videos

Role of Functional Groups
Organic Chemistry Functional Groups
The manner in which the functional groups indulge in a chemical reaction can be further modified with the help of other functional groups, and these groups can also be interconverted.
A few functional groups involving carbon are illustrated below.
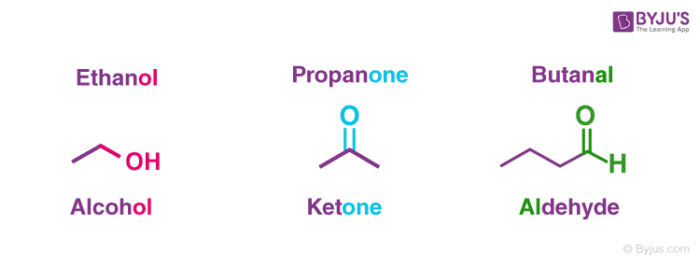
Therefore, it can be understood that functional groups are the moieties which exhibit their own distinct features and properties independent of the molecule they are attached to.
- Covalent bonding links the atoms of these groups and the group as a whole to the molecule.
- In the case of polymers, the functional groups are generally attached to the nonpolar core of the carbon atoms in each repeating unit of the corresponding polymer, infusing the carbon chain with specific chemical characteristics.
- Some functional groups have an ionic charge on them, as observed in carboxylate salts containing the -COO– ionic group.
- When these groups are attached to molecules, they convert the molecule into either complexions or polyatomic ions.
- In a coordination complex, the functional group that is bound to the central atom is said to be a ligand.
Some more functional groups containing elements such as nitrogen and oxygen featuring different hybridizations of the carbon-nitrogen and the carbon-oxygen bonds are illustrated below.
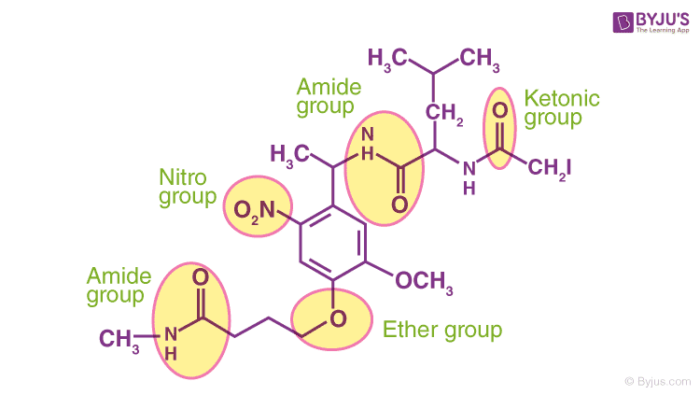
The presence of functional groups in a molecule also affects the solubility and the tendency to form complexes of the molecule in question. If the functional groups of the solute and the solvent interact well, the solubility increases. For example, since sugar and water both contain the -OH (hydroxyl) group, sugar can be easily dissolved in water.
In the scenario wherein a highly electronegative functional group is attached to a less electronegative atom or molecule, a polarity arises which enables the initially nonpolar molecule to be soluble in water or other aqueous environments.
Nomenclature of Common Functional Groups
The common functional groups, along with the prefix and the suffix which must be used in their nomenclature is provided in this subsection. Additionally, a brief description of the constitution of each of these groups is also provided.
1. Hydrocarbons
- Alkanes, alkenes, and alkynes (and sometimes the derivatives of benzene) are represented by the symbol R. these groups are also referred to as hydrocarbyl groups since they contain only carbon and hydrogen atoms. However, they may vary in the types of bonds between two carbon atoms, such as double or triple bonds.
- The reactivity of these groups varies due to the nature of the carbon-carbon bond. Some groups are made up of a long, branched alkane or a ring-structured alkane, which are assigned specific names. Examples include names such as bornyl and cyclohexyl.
- The hydrocarbon functional groups may have an ionic charge on them. The positively charged structures are referred to as carbocations whereas the negatively charged hydrocarbons are called carbanions.
2. Haloalkanes
- Haloalkanes, or alkyl halides, are the functional groups which contain a bond between a carbon atom and a halogen. The prefix used to denote a halogen is ‘halo-’. For example, the compound CH3F can be called fluoromethane, and the prefix here is fluoro.
- The suffix used to denote a halogen is the ‘halide’. For example, the same compound, fluoromethane (CH3F) can also be referred to as methyl fluoride, the suffix being fluoride.
- The carbon-halogen bond varies in strength and stability based on the halogen. For example, the carbon-iodine bond in alkyl iodides is quite weak but the carbon-fluorine bond in alkyl fluorides is quite strong and stable.
- Excluding these alkyl fluorides, all the alkyl halides readily undergo elimination reactions or nucleophilic substitution reactions.
3. Oxygen-Containing Functional Groups
- The properties of the functional groups containing a carbon-oxygen bond are entirely dependant on the hybridization of the carbon-oxygen bond.
- This can be explained by the electron donating effect of the sp3 hybridization of oxygen which can be observed in alcohols in sharp contrast with the electron withdrawing effect of the sp2 hybridized oxygen which can be observed in the carbonyl groups which contain a carbon-oxygen double bond.
- The suffixes used in the nomenclature of compounds which have a functional group containing a C-O bond are tabulated below along with examples.
It can be noted that only a few, common functional groups containing the carbon-oxygen bond are tabulated above. Many uncommon groups with complex compositions such as acetal groups (RCH(OR’)(OR’’), or ketal groups RC(OR’)(OR”)R”. Nitrogen-Containing Functional Groups. The substituent groups that contain nitrogen may also contain carbon-oxygen bonds.
For example, the amide functional group has the formula R-(CO)-NR2 and therefore has a carbonyl carbon which is bonded to a nitrogen atom, which is in turn bonded to two other alkyl groups. Some common functional groups that contain nitrogen are tabulated below along with the suffixes for their nomenclature. It can be noted that many nitrogen-containing functional groups with comparatively large sizes have not been mentioned in the tabular column given above, the pyridine derivatives with the formula RC5H4N, for example.
| Functional Group and Formula | Suffix | Example |
| Alcohol, R-OH | -ol | Methanol |
| Ketone, R-(CO)-R’ | -one | Butanone |
| Aldehyde, R-CHO | -al | Ethanal (acetaldehyde) |
| Acyl Halide, R-(CO)-X | -oyl halide | Ethanoyl Chloride (acetyl chloride) |
| Carboxylate, R-COO– | -oate | Sodium Ethanoate (sodium acetate) |
| Carboxylic Acid, R-COOH | -oic acid | Ethanoic Acid (acetic acid) |
| Ester, R-(CO)-O-R’ | Alkyl alkanoate | Ethyl Butanoate (Ethyl butyrate) |
| Ether, R-O-R’ | Alkyl ether | Diethyl Ether (ethoxyethane) |
| Functional Group and Formula | Suffix | Example |
| Amide, R-(CO)-N-R2 | -amide | Ethanamide (acetamide) |
| Primary Amine, R-NH2 | -amine | Methylamine |
| Secondary Amine, R2NH | -amine | Dimethylamine |
| Tertiary Amine, NR3 | -amine | Trimethylamine |
| Imide, (RCO)2N-R’ | -imide | Succinimide |
| Cyanate, R-OCN | Alkyl cyanate | Methyl Cyanate |
| Isocyanate, R-NCO | Alkyl isocyanate | Methyl Isocyanate |
Frequently Asked Questions – FAQs
What is a functional group example?
A functional group in organic chemistry is a collection of atoms within molecules which bind together to react in predictable ways. Examples of functional groups include the group hydroxyl, ketone, amine, and ether.
How do you classify functional groups?
The first carbon atom attached to the functional group is called alpha carbon; the second, beta carbon; the third, gamma carbon, and so forth. Similarly, a functional group can be called principal, secondary, or tertiary, depending on whether it is connected to one, two , or three atoms of carbon.
What are the four functional groups?
In biological molecules, some of the essential functional groups include hydroxyl, methyl, carbonyl, carboxyl, amino, phosphate, and sulfhydryl groups. These groups play a significant role in forming molecules such as DNA, proteins, carbohydrates, and lipids.
What do functional groups do?
For organic chemistry, functional groups are unique groups of atoms within molecules, which are responsible for those molecules’ characteristic chemical reactions. No matter the size of the molecule, the same functional group will undergo the same or identical chemical reaction(s).
What is called a functional group?
Functional groups are groups of one or more atoms with distinctive chemical properties regardless of what is attached to them. The atoms of functional groups are bound by covalent bonds with one another and with the rest of the molecule. Functional groups in a coordination complex which bind to a central atom are called ligands.
How do you identify a functional group?
The groups of atoms which are bound to the carbon backbone of organic molecules are functional groups. The signature chemical reactions of organic compounds are responsible for functional groupings. They are less stable and more likely to partake in chemical reactions than the carbon backbone.
Is alkene a functional group?
Chemical compounds containing only carbon and hydrogen are hydrocarbons. These contain alkanes, alkenes, aromatics and alkynes. A carbon-carbon double bond is a functional group inside an alkene. In an alkyne, the functional group is a triple carbon-carbon bond.
What is a carboxy group?
A group of carboxyls is a typical group of functions shown in chemistry. A group of carboxyls is classified as having a group of carbonyls and hydroxyls all bound to a carbon atom. A carbonyl group is a carbon double-bonded to oxygen in order to update the mind, and an OH group is a hydroxyl group.
Are all functional groups polar?
What is haloalkane?
To learn more about functional groups, including functional groups that contain sulfur, phosphorus, or boron, register with BYJU’S and download the mobile app on your smartphone.

Comments