What is Tartaric Acid?
Tartaric Acid is a carboxylic acid with a chemical formula C4H6O6.
Tartaric Acid is an organic acid found in many vegetables and fruits such as bananas, and grapes, but also in bananas, citrus, and tamarinds. It is also known as 2,3-dihydroxysuccinic acid or Racemic acid. It is used to generate carbon dioxide. It is a diprotic aldaric acid which is crystalline white. Baking powder is a mixture of tartaric acid with sodium bicarbonate.
It is widely used in the field of pharmaceuticals. High doses of tartaric acid can lead to paralysis or death.
Table of Contents
- Properties of Tartaric acid
- Tartaric Acid Structure
- Uses of Tartaric Acid
- Frequently Asked Questions
Properties of Tartaric Acid – C4H6O6
| C4H6O6 | Tartaric Acid |
| Molecular Weight/ Molar Mass | 150.087 g/mol |
| Density | 1.79 g/mL |
| Boiling Point | 275 °C |
| Melting Point | 171 to 174 °C |
Tartaric Acid Structure – C4H6O6
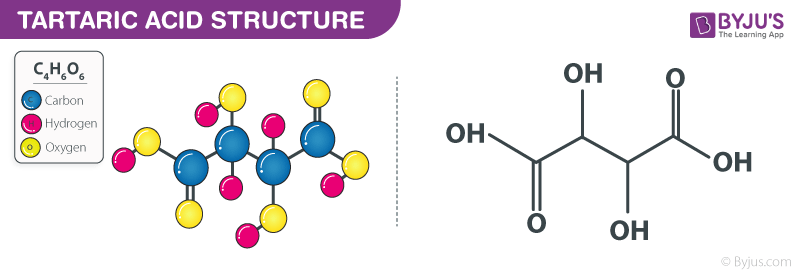
Tartaric Acid Structure
Uses of Tartaric Acid
- It is used to improve the taste of oral medications
- It is used to chelate metal ions such as magnesium and calcium
- It is used in recipes as a leavening agent along with baking soda
- It is used as an antioxidant
- It is as one of the important acids in wine
- It is used in foods to give a sour taste
- It is sometimes used to induce vomiting
- It is used to make silver mirrors
- In its ester form, it is used in the dyeing of textiles
- It is used in the tanning of leather
- It is used in candies
- In its cream form, it is used as a stabilizer in food
Frequently Asked Questions
Is tartaric acid a strong acid?
It is a stronger acid than malic and citric acid, and less susceptible to microorganism breakdown during alcoholic and malolactic fermentations. When tartaric acid reacts with potassium sulfate, the potassium salt of tartaric acid is obtained which has less solubility in water.
What is tartaric acid used for?
Tartaric acid is an essential food additive that is usually mixed with baking soda in recipes to serve as a leavening agent. It can be used in all food types except untreated foods. Tartaric acid occurs naturally in plants such as grapes, apricots, oranges, bananas, avocados, and tamarinds.
What is the difference between tartaric acid and citric acid?
The key distinction between tartaric acid and citric acid is that tartaric acid occurs naturally in grapes while citric acid occurs naturally in citrus fruits. Tartaric acid and citric acid are two types of plant acids which are used as natural additives to food. Tartaric acid as well as citric acid, are also antioxidants. Tartaric acid is diprotic where as citric acid is triprotic.
Is tartaric acid strong or weak?
Most organic acids are weak acids. Tartaric acid is also a weak acid which ionises partially in water.
What is tartaric acid found in?
Tartaric acid is an organic white diprotic, crystalline acid. The compound naturally occurs in many plants, particularly in grapes, bananas, and tamarinds. It is also one of the wine’s primary acids.
Also, Read:
| Benzoic Acid | Acetic Acid |
| Sodium Hydroxide | Sulfuric Acid |
Register with BYJU’S to learn more about the properties and importance of tartaric acid along with the structure of C4H6O6 from chemistry experts.

Comments