In order to understand the hybridization of CH4 (methane), we have to take a look at the atomic orbitals which are of different shape and energy that take part in the process. The type of hybridization involved with CH4 is sp3. We will discuss in detail how this hybridization occurs below.
| Name of the Molecule | Methane |
| Molecular Formula | CH4 |
| Hybridization Type | sp3 |
| Bond Angle | 109.5o |
| Geometry | Tetrahedral |
What is the Hybridization of Methane?
When we talk about CH4 it is basically a combination of 1 carbon and 4 hydrogen atoms. However, to form this compound the central atom carbon which has 4 valence electrons obtain more electrons from 4 hydrogen atoms to complete its octet. When the electrons are shared between carbon and hydrogen there is a formation of a covalent bond or bonds to be more accurate.
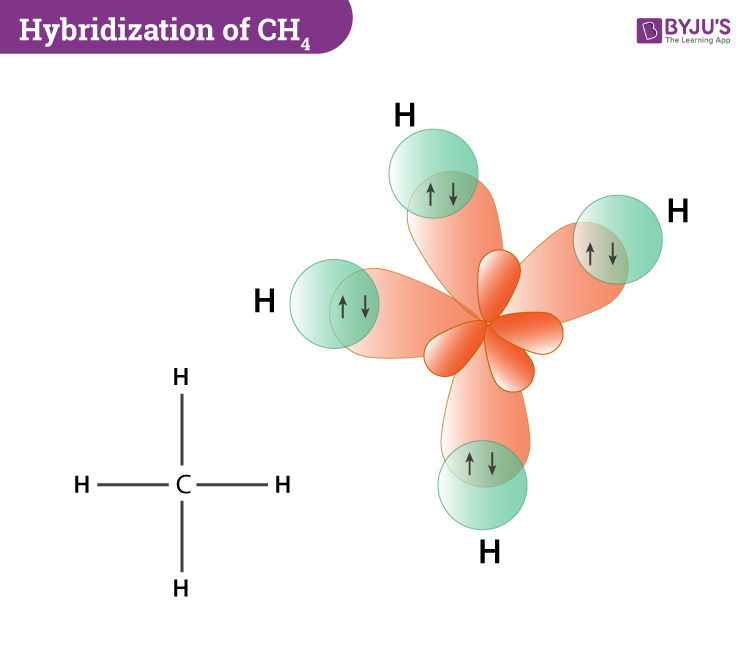
Now coming to the hybridization of methane, the central atom carbon is sp3 hybridized. This is because one 2s orbital and three 2p orbitals in the valence shell of carbon combine to form four sp3 hybrid orbitals which are of equal energy and shape. Further, four H atoms also use these four sp3 hybrid orbitals of carbon to form C-H sigma bonds which ultimately leads to the formation of the methane molecule.
Important Points To Remember
- Each sp3 hybrid orbital of carbon overlaps 1s-orbital of hydrogen to C-H sigma bonds.
- The hybridization involves the mixing of 1 s orbital and 3 p orbitals and there are no lone pairs.
- The sp3 hybrid orbitals are of equal energy and shape. They contain one unpaired electron each.
CH4 Molecular Geometry And Bond Angles
We have already discussed the bond formation and hybridization process above. Determining CH4 molecular geometry should be easier. In methane, the four hybrid orbitals are located in such a manner so as to decrease the force of repulsion between them. Nonetheless, the four orbitals do repel each other and get placed at the corners of a tetrahedron. CH4 has a tetrahedral shape. The sp3 hybrid orbitals have a bond angle of 109.5o.
Read More About Hybridization of Other Chemical Compounds
- Hybridization Of C2H2
- Hybridization Of SF6
- Hybridization Of NO3
- Hybridization Of CH4
- Hybridization Of Benzene
Chemical Bonding

Comments