Students will find the explanation of hybridization of C2H2 (ethyne) on this page. The type of hybridization that exists in this chemical compound is sp type. To understand the process students have to learn about the bonding and the orbitals. We will discuss everything in detail below.
| Name of the Molecule | Acetylene or Ethyne |
| Molecular Formula | C2H2 |
| Hybridization Type | sp |
| Bond Angle | 180o |
| Geometry | Linear |
What is the Hybridization of Ethyne?
When we break down ethyne molecules it basically consists of 2 CH molecules. However, we will take first take both carbon and hydrogen molecule separately and draw their orbital diagrams. When we do this we will see that carbon has 6 electrons and hydrogen has one electron.
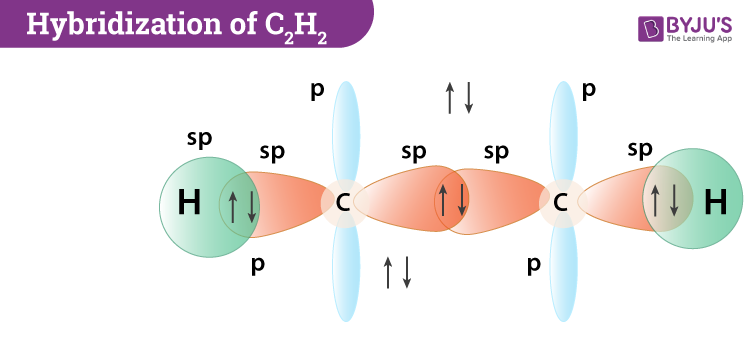
Now, if we see the electronic configuration of carbon in its ground state it will be represented as 1s2 2s2 2p2. When it gets into an excited state, one of the electron from 2s orbital will move or jump to the 2pz orbital and the electronic configuration will change to 1s2 2s1 2px12py1 2pz1. Meanwhile, the CH molecule has only 1 hydrogen atom, therefore the 2s1 and the 2pz1 orbitals get hybridised. This further leads to the formation of 4 sp hybridized orbitals wherein each CH molecule will form 2 hybridized sp orbitals.
During hybridization, C-C sigma bond is formed when one sp orbital overlaps from each of the carbons and two C-H bonds are created when second sp orbital on each carbon overlaps with 1s orbital of hydrogen. In this, the carbon atom will have two half-filled 2p orbitals. These two pairs of p orbitals do not participate in the hybridization and instead form two pi bonds resulting in the creation of a triple bond.
Important Points To Remember
- In the formation of C2H2, the carbon atom needs extra electrons to form 4 bonds with hydrogen and other carbon atoms. As a result, one 2s2 pair is moved to the empty 2pz orbital.
- The 2s orbital in each carbon hybridizes with one of the 2p orbitals and forms two sp hybrid orbitals.
- Ethyne has a triple bond between the two carbon atoms.
C2H2 Molecular Geometry And Bond Angles
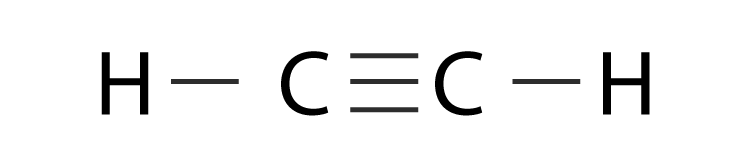
As a result of the double bond C2H2 molecular geometry is linear with a bond angle of 180o.
Read More About Hybridization of Other Chemical Compounds
- Hybridization Of XeF4
- Hybridization Of SF4
- Hybridization Of PCl3
- Hybridization Of Graphite
- Hybridization Of SO3
Chemical Bonding

Thank you for clearing my doubts