
1) 3-Bromo-5-methyl cyclopentane carboxylic acid
2) 4-Bromo-2-methyl cyclopentane carboxylic acid
3) 5-Bromo-3-methyl cyclopentanoic acid
4) 3-Bromo-5-methyl cyclopentanoic acid
Answer: (2)

4–Bromo-2-methyl cyclopentane carboxylic acid
1) +3
2) +4
3) +2
4) +5
Answer: (1)
Pb(NO3)2 → PbO + NO2 + O2
(A)
Brown gas
2NO2 → N2O4 [on cooling]
(C)
colourless solid

(C)
blue solid
1) F– > O2– > Na+ > Mg2+
2) Mg2+ > Na+ > F– > O2–
3) O2– > F– > Na+ > Mg2+
4) O2– > F– > Mg2+ > Na+
Answer: (3)
O2– > F– > Na+ > Mg2+

Answer: (3)

1) Microwave
2) Infrared
3) Ultraviolet
4) Visible
Answer: (4)
The question should be a bonus as lines of the Balmer series belong to both UV as well as visible regions of the EM spectrum. However most appropriate should be the visible region.

1) If Eext = 1.1 V, no flow of e– or current occurs
2) If Eext > 1.1 V, Zn dissolves at Zn electrode and Cu deposits at Cu electrode
3) If Eext > 1.1 V, e– flow from Cu to Zn
4) If Eext < 1.1 V, Zn dissolves at the anode and Cu deposits at the cathode
Answer: (2)
1) One acetal and one hemiacetal
2) One acetal and one ketal
3) One ketal and one hemiketal
4) Two acetals
Answer: (1)

Question 8: Match the following:
| (i) Foam | (a) smoke |
| (ii) Gel | (b) cell fluid |
| (iii) Aerosol | (c) jellies |
| (iv) Emulsion | (d) rubber |
| (e) froth | |
| (f) milk |
1) (i)-(e), (ii)-(c), (iii)-(a), (iv)-(f)
2) (i)-(b), (ii)-(c), (iii)-(e), (iv)-(d)
3) (i)-(d), (ii)-(b), (iii)-(a), (iv)-(e)
4) (i)-(d), (ii)-(b), (iii)-(e), (iv)-(f)
Answer: (1)
Foam → Froth, whipped cream, soap lather
Gel → Cheese, butter, jellies
Aerosol → smoke dust
Emulsion → milk
Sol → Cell fluid
rubber → Solid form
froth → form
(i) – e, (ii) – c, (iii) – a, (iv) – f

Question 10: Among the statements (a)-(d), the correct ones are:
(a) Limestone is decomposed to CaO during the extraction of iron from its oxides.
(b) In the extraction of silver, silver is extracted as an anionic complex.
(c) Nickel is purified by Mond’s process.
(d) Zr and Ti are purified by Van Arkel method.
1) (c) and (d) only
2) (b), (c) and (d) only
3) (a), (b), (c) and (d)
4) (a), (c) and (d) only
Answer: (3)
Limestone finally goes to slag formation
CaCO3 → CaO + CO2
CaO + SiO2 → CaSiO3
Slag
Question 11: For one mole of an ideal gas, which of these statements must be true?
(a) U and H each depends only on temperature
(b) Compressibility factor z is not equal to 1
(c) CP,m – CV,m = R
(d) dU = CV dT for any process
1) (a), (c) and (d)
2) (a) and (c)
3) (c) and (d)
4) (b), (c) and (d)
Answer: (1)
For ideal gas
𝜹v / 𝜹v|T = 0 & 𝜹H / 𝜹v|T = 0
(a) Hence function of temperature only.
(b) Compressibility factor (z) = 1 Always
(c) Cp,m – CV,m = R
(d) dv = nCv,m dT for all process
The answers are a, c, d.


Answer: (2)
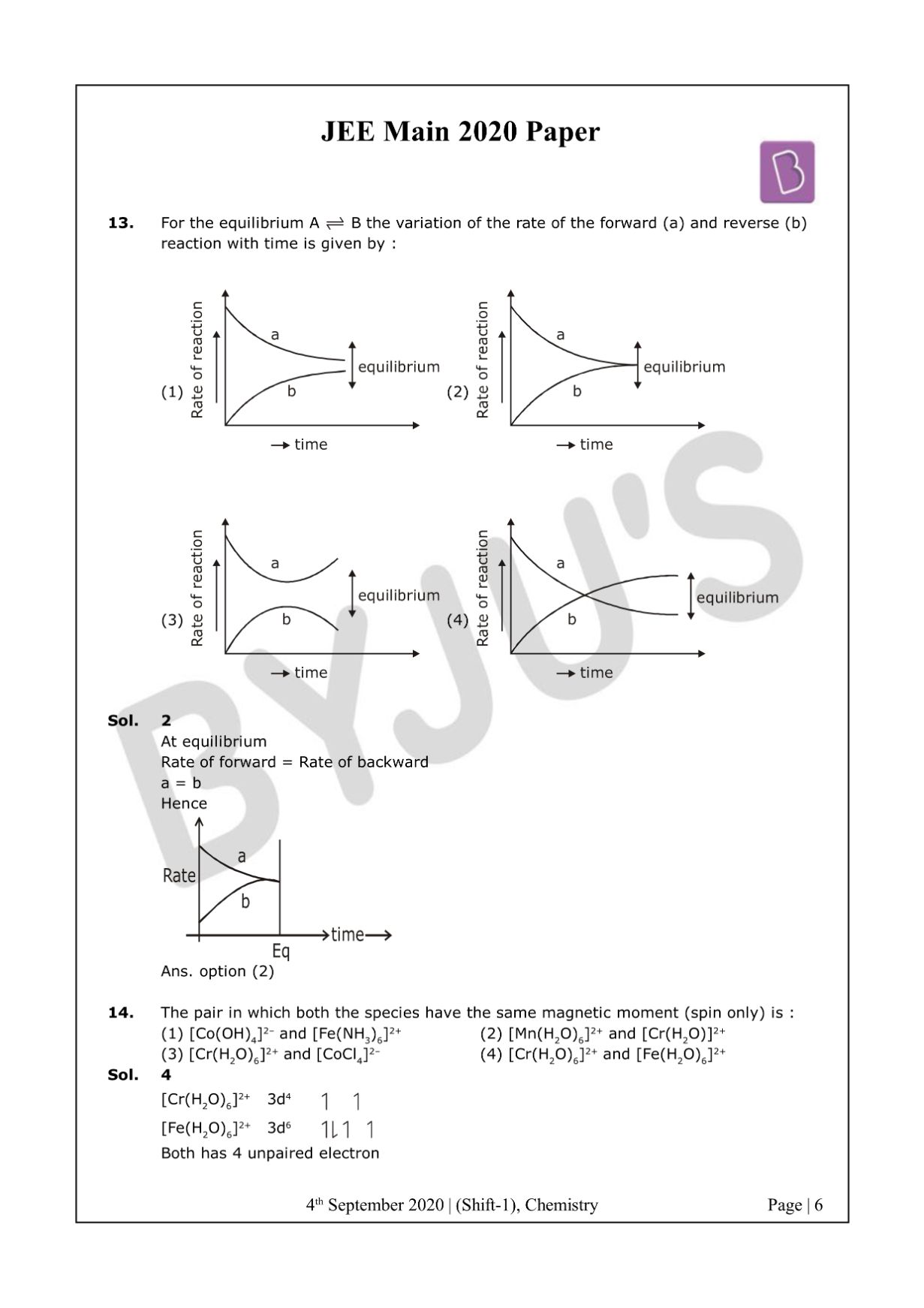
At equilibrium
Rate of forward = Rate of backward
a = b
Hence

The answer is option 2.
1) [Co(OH)4]2– and [Fe(NH3)6]2+
2) [Mn(H2O)6]2+ and [Cr(H2O)]2+
3) [Cr(H2O)6]2+ and [CoCl4]2–
4) [Cr(H2O)6]2+ and [Fe(H2O)6]2+
Answer: (4)

Both have 4 unpaired electrons.
1) 2
2) 3
3) 4
4) 1
Answer: (2)
Three linkage isomers:
[Pt(en)(NO2)2] [Pt(en)(ONO)2] [Pt(en)(NO2)(ONO)]Question 16: The decreasing order of reactivity of the following organic molecules towards the AgNO3 solution is:

1) (B) > (A) > (C) > (D)
2) (A) > (B) > (C) > (D)
3) (A) > (B) > (D) > (C)
4) (C) > (D) > (A) > (B)
Answer: (1)

Order or stability
(B) > (A) > (C) > (D)
Question 17: The intermolecular potential energy for the molecules A, B, C and D given below suggests that:

1) A–A has the largest bond enthalpy.
2) D is more electronegative than other atoms.
3) A–D has the shortest bond length.
4) A–B has the stiffest bond.
Answer: (4)
As EA–B is the highest, the answer is option 4.
1) Thymine and proline
2) Adenine and thymine
3) Adenine and lysine
4) Adenine and proline
Answer: (3)
CHCl3 + Alc. KOH reacts with those compounds which have a –NH2 group.

1) Actinoids and Group 6
2) Group 11 and Group 4
3) Group 6 and Actinoids
4) Actinoids and Group 4
Answer: (4)

The answer is Actinoids & 4th group.
1) Li2O2, Na2O2 and K2O2
2) Li2O, Na2O2 and KO2
3) Li2O, Na2O and K2O2
4) Li2O, Na2O2 and K2O
Answer: (2)
Li2O, Na2O2 and KO2
Question 21: The number of chiral centres present in [B] is ________.

Answer: (4)

4 chiral centres are present in the final product.
Answer: 600

Answer: 3400
N2 + 3H2 → 2NH3
2800g 1000g
100 mol 500 mol
L.R. mole of NH3 produced = 200 mol
mass = 3400 g
Answer: 60
t75% = 90 min = 2 × t1/2
t1/2 = 45 min
[ln (2) / 45] × 60% = ln [100 / 40]t60% = 45 × [0.4 / 0.3]
t60% = 60 min
Answer: 85
H2O2 + KMnO4 → Mn+2 + O2
[moles of H2O2] × 2 = [0.316 / 158] * 5moles of H2O2 = 5 × 10–3
mass of H2O2 = 170 × 10–3 g
% purity = {[170 * 10-3] / 0.2} * 100 = 85%
Video Lessons – September 4 Shift 1 Chemistry





JEE Main 2020 Chemistry Paper With Solutions Shift 1 September 4









Comments