A detailed description of the hybridization of SO3 is provided on this page along with its molecular geometry. SO3 has a sp2 type of hybridization. We will understand how the molecule obtains such hybridized state below.
| Name of the Molecule | Sulphur Trioxide |
| Molecular Formula | SO3 |
| Hybridization Type | sp2 |
| Bond Angle | 120o |
| Geometry | Trigonal Planar |
What is the Hybridization of Sulphur Trioxide?
SO3 is a combination of two elements one Sulphur and three Oxygen atoms. To understand the hybridization of sulphur trioxide we have to understand the bonding between sulphur and oxygen. If we draw and look at the Lewis structure sulphur will be the central atom and will have three double bonds with oxygen. In this, there is one sigma and one pi bond formed. This gives us the sp2 hybridization type.

We can also say that during bonding the d orbitals on the sulphur atom will overlap with p orbitals of the oxygen atoms. Meanwhile, during hybridization, the one s and two p orbitals are hybridized where one of the sets of electrons is a nonbonding lone pair.
Important Points To Remember
- In SO3 sulphur will be the central atom and will have three double bonds with oxygen.
- The sigma bond (single bond) is between the sp2 orbital on Sulphur and another sp2 on Oxygen.
- The double bond is created between the empty p orbital of Sulphur and an empty p orbital of Oxygen.
SO3 Molecular Geometry And Bond Angles
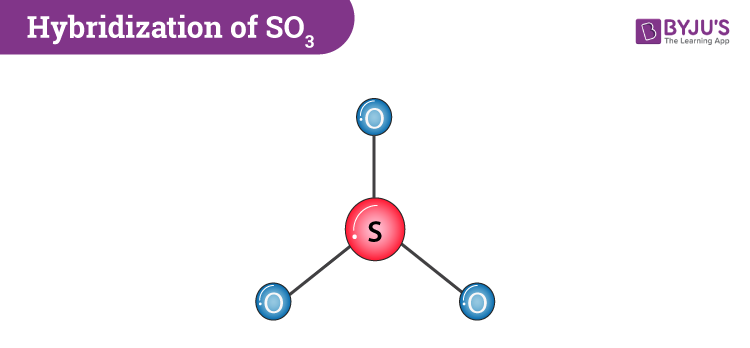
If we look at the SO3 molecular geometry it is trigonal planar with symmetric charge distribution around the central atom. Sulphur trioxide is also non-polar. It has a bond angle of 120o.
Read More About Hybridization of Other Chemical Compounds
- Hybridization Of C2H2
- Hybridization Of SF6
- Hybridization Of NO3
- Hybridization Of CH4
- Hybridization Of Benzene
Chemical Bonding

Comments