What Is Hydrogen Bonding?
Hydrogen bonding refers to the formation of hydrogen bonds, which are a special class of attractive intermolecular forces that arise due to the dipole-dipole interaction between a hydrogen atom that is bonded to a highly electronegative atom and another highly electronegative atom which lies in the vicinity of the hydrogen atom. For example, in water molecules (H2O), hydrogen is covalently bonded to the more electronegative oxygen atom. Therefore, hydrogen bonding arises in water molecules due to the dipole-dipole interactions between the hydrogen atom of one water molecule and the oxygen atom of another H2O molecule.
Download Complete Chapter Notes of Chemical Bonding and Molecular Structure
Download Now
Here, the location of the bond pair of electrons in the O-H bond is very close to the oxygen nucleus (due to the large difference in the electronegativities of oxygen and hydrogen). Therefore, the oxygen atom develops a partial negative charge (-δ), and the hydrogen atom develops a partial positive charge (+δ). Now, hydrogen bonding can occur due to the electrostatic attraction between the hydrogen atom of one water molecule (with +δ charge) and the oxygen atom of another water molecule (with -δ charge). Thus, hydrogen bonds are a very special class of intermolecular attractive forces that arise only in compounds featuring hydrogen atoms bonded to a highly electronegative atom. Hydrogen bonds are mostly strong in comparison to normal dipole-dipole and dispersion forces. However, they are weak compared to true covalent or ionic bonds.
Table of Content
What Are the Conditions for Hydrogen Bonding?
In a molecule, when a hydrogen atom is linked to a highly electronegative atom, it attracts the shared pair of electrons more, and so this end of the molecule becomes slightly negative while the other end becomes slightly positive. The negative end of one molecule attracts the positive end of the other, and as a result, a weak bond is formed between them. This bond is called the hydrogen bond.
As a result of hydrogen bonding, a hydrogen atom links the two electronegative atoms simultaneously, one by a covalent bond and the other by a hydrogen bond. The conditions for hydrogen bonding are as follows:
- The molecule must contain a highly electronegative atom linked to the hydrogen atom. The higher the electronegativity, the more the polarization of the molecule.
- The size of the electronegative atom should be small. The smaller the size, the greater the electrostatic attraction.
Also Read:
Effects of Hydrogen Bonding on Elements
Association
The molecules of carboxylic acids exist as dimer because of the hydrogen bonding. The molecular masses of such compounds are found to be double than those calculated from their simple formula.
Dissociation
In an aqueous solution, HF dissociates and gives the difluoride ion instead of the fluoride ion. This is due to hydrogen bonding in HF. The molecules of HCl, HBr and HI do not form a hydrogen bond. This explains the non-existence of compounds like KHCl2, KHBr2 and KHI2.
Why do compounds having hydrogen bonding have high melting and boiling points?
Compounds having hydrogen bonding show abnormally high melting and boiling points. The high melting and boiling point of the compound containing hydrogen bonds is due to the fact that some extra energy is needed to break these bonds.
- The unusually high boiling point of hydrogen fluoride among the halogen acid is due to the existence of hydrogen bonding.
- H2O is a liquid, whereas H2S, H2Se and H2Te are all gases at ordinary temperatures. In water, hydrogen bonding causes linkages in the water molecules which result in the boiling point of water being more than that of the other compounds.
- Ammonia has a higher boiling point than PH3 because there is hydrogen bonding in NH3 but not in PH3.
- Ethanol has a higher boiling point than diethyl ether because there is hydrogen bonding in the ethanol.
Examples of Hydrogen Bonding
Hydrogen Bonding in Hydrogen Fluoride
Fluorine, having the highest value of electronegativity, forms the strongest hydrogen bond.

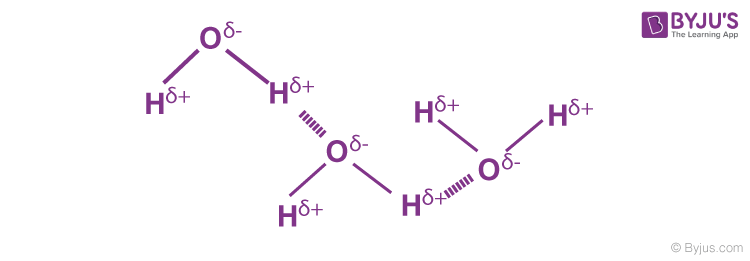
Hydrogen Bonding in Water
A water molecule contains a highly electronegative oxygen atom linked to the hydrogen atom. The oxygen atom attracts the shared pair of electrons more, and this end of the molecule becomes negative, whereas the hydrogen atoms become positive.
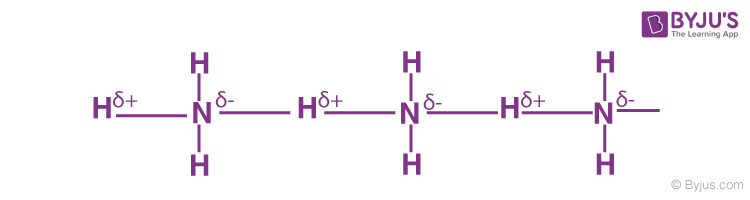
Hydrogen Bonding in Ammonia
It contains highly electronegative atom nitrogen linked to hydrogen atoms.
Hydrogen Bonding in Alcohols and Carboxylic Acid
Alcohol is a type of organic molecule which contains an -OH group. Normally, if any molecule which contains the hydrogen atom is connected to either oxygen or nitrogen directly, then hydrogen bonding is easily formed.

Hydrogen Bonding in Alcohols

Hydrogen Bonding in Carboxylic Acid
Hydrogen Bonding in Polymers
Hydrogen bonding is an important factor in determining the 3D structures and properties that are acquired by synthetic and natural proteins. Hydrogen bonds also play an important role in defining the structure of cellulose as well as derived polymers such as cotton or flax.
Strength of the Hydrogen Bond
The hydrogen bond is a weak bond. The strength of the hydrogen bond is in-between the weak Van der Waals forces and the strong covalent bonds.
The dissociation energy of the hydrogen bond depends upon the attraction of the shared pair of electrons, and hence on the electronegativity of the atom.
Properties of Hydrogen Bonding
- Solubility: Lower alcohols are soluble in water because of the hydrogen bonding which can take place between water and alcohol molecules.
- Volatility: As the compounds involving hydrogen bonding between different molecules have a higher boiling point, they are less volatile.
- Viscosity and surface tension: The substances which contain hydrogen bonding exist as associated molecules. So, their flow becomes comparatively difficult. They have higher viscosity and high surface tension.
- The lower density of ice than water: In the case of solid ice, hydrogen bonding gives rise to a cage-like structure of water molecules. As a matter of fact, each water molecule is linked tetrahedral to four water molecules. The molecules are not as closely packed as they are in a liquid state. When ice melts, this case-like structure collapses, and the molecules come closer to each other. Thus for the same mass of water, the volume decreases and density increases. Therefore, ice has a lower density than water at 273 K. That is why ice floats.
Related Articles
Types of Hydrogen Bonding
There are two types of H bonds, and it is classified as the following:
- Intermolecular Hydrogen Bonding
- Intramolecular Hydrogen Bonding
Intermolecular Hydrogen Bonding
When hydrogen bonding takes place between different molecules of the same or different compounds, it is called intermolecular hydrogen bonding.
For example, hydrogen bonding in water, alcohol, ammonia etc.
Intramolecular Hydrogen Bonding
The hydrogen bonding which takes place within a molecule itself is called intramolecular hydrogen bonding.
It takes place in compounds containing two groups such that one group contains a hydrogen atom linked to an electronegative atom, and the other group contains a highly electronegative atom linked to a lesser electronegative atom of the other group.
The bond is formed between the hydrogen atoms of one group with the more electronegative atom of the other group.
Problem: Indicate which of the following molecules could form hydrogen bonds with other like molecules.
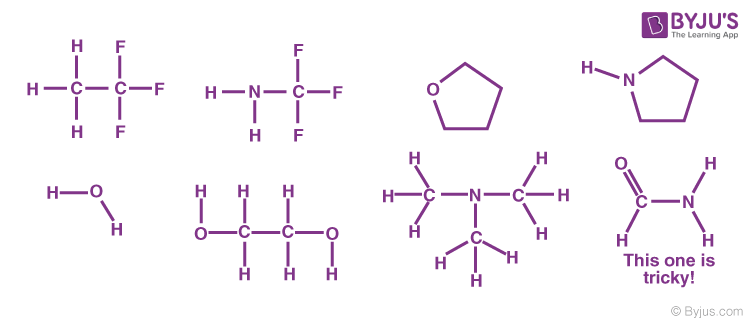
Symmetric Hydrogen Bond
This is a special type of hydrogen bond where the proton is usually placed in the middle between two identical atoms. The strength of the bond between each atom is equal. The symmetric hydrogen bond is a type of three-centre four-electron bond. This bond is also much stronger compared to the “normal” hydrogen bond, and its strength is almost similar to a covalent bond.
What Is Metallic Bonding?
Metals are characterised by bright, lustre, high electrical and thermal conductivity, malleability, ductility and high tensile strength. A metallic crystal consists of a very large number of atoms arranged in a regular pattern.
Different models have been proposed to explain the nature of metallic bonding. The two most important modules are as follows.

Electron Sea Model
In this model, a metal is assumed to consist of a lattice of positive ions (or kernels) immersed in a sea of mobile valence electrons, which move freely within the boundaries of a crystal. A positive kernel consists of the nucleus of the atom together with its core on a kernel is, therefore, equal in magnitude to the total valence electronic charge per atom.
The free electrons shield the positively charged ion cores from mutual electrostatic repulsive forces which they would otherwise exert upon one another. In a way, these free electrons act as ‘glue’ to hold the ion cores together.
The forces that hold the atoms together in a metal as a result of the attraction between positive ions and surrounding freely mobile electrons are known as metallic bonds.
Through the electron sea predated quantum mechanics, it still satisfactorily explains certain properties of the metals. The electrical and thermal conductivity of metals, for example, can be explained by the presence of mobile electrons in metals.
On applying an electron field, these mobile electrons conduct electricity throughout the metals from one end to another. Similarly, if one part of the metal is heated, the mobile electrons in the part of the metal acquire a large amount of kinetic energy. Being free and mobile, these electrons move rapidly throughout the metal and conduct heat to the other part of the metal.
Hydrogen Bonding


Comments