Biomolecules NEET Important Notes
Find below the important notes for the chapter, Biomolecules, as per NEET Biology syllabus. This is helpful for aspirants of NEET and other exams during last-minute revision. Important notes for NEET Biology- Biomolecules covers all the important topics and concepts useful for the exam. Check BYJU’S for the full set of important notes and study material for NEET Biology and solve the NEET Biology MCQs to check your understanding of the subject.
Download Complete Chapter Notes of Biomolecules
Download Now
| Name of the NEET sub-section | Topic | Notes helpful for |
| Biology | Biomolecules | NEET exams |
Biomolecules – Important Points, Summary, Revision, Highlights
ProteinsCarbohydratesLipidsNucleic AcidsDNARNAEnzymes
Biomolecules
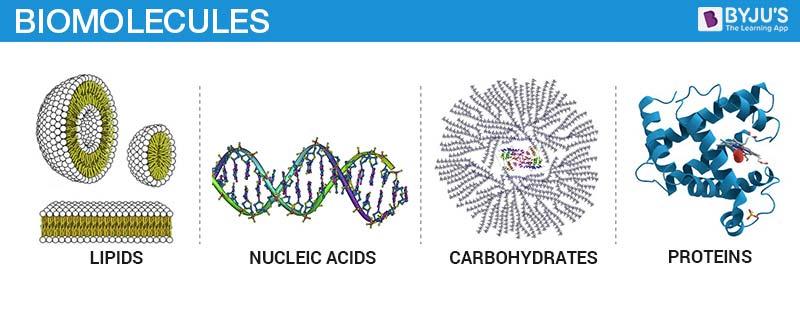
Biomolecules include both micromolecules, e.g. amino acids, nitrogenous bases, fatty acids, sugar, etc. and macromolecules, such as carbohydrates, proteins, lipids and nucleic acids.
Living organisms are made up of organic as well as inorganic substances. For analysing the organic constituents of living tissue, we mix it with CCl3COOH (Trichloroacetic acid) and make a slurry by grinding. We get two fractions:
Acid soluble fraction- Organic micromolecules (biomolecules) (mol wt. 18-800 Da) and inorganic compounds, e.g. phosphate, sulphate, etc. It mostly accounts for the cytoplasm
Acid insoluble fraction- Polymeric macromolecules (mol wt. >10 thousand Da) and also lipid (a component of the cell membrane and form water-insoluble vesicles on fragmentation)
The inorganic constituents can be estimated by analysing the ash formed after burning a tissue completely.
All the macromolecules are homo or heteropolymers of various simple compounds.
Do Check: NEET 2022 Answer Key With Solutions
Protein- Polymer of amino acids
Carbohydrates (Polysaccharides)- Polymer of simple sugars, e.g. glucose, fructose
Fats- Fatty acids and glycerol
Nucleic acids- Nitrogenous bases, sugar and phosphate
Water constitutes 70-90% of cells. Proteins are 10-15%, nucleic acids are 5-7%, carbohydrates are 3%, lipids are 2% and the rest 1% are ions.
Amino acids, fatty acids, sugars, etc. are called primary metabolites, which take part in the physiological processes. Other than these, there are varied compounds present in the cells of plants, microbes and fungi, which are called secondary metabolites. Some of the secondary metabolites have ecological significance.
Secondary metabolites can be grouped under various categories:
Pigments (anthocyanins, carotene, etc.), drugs (curcumin, vinblastine, etc.), gum, rubber, toxins (ricin, abrin), alkaloids (codeine, morphine), essential oils, terpenoids, lectins (concanavalin A), etc.
Proteins
Proteins perform many vital functions in the living organisms. Proteins are present as enzymes (trypsin, lipases, amylase, etc.), hormones (Insulin), tissue fibres are made up of proteins (collagen, elastin, etc.). Some of the protein molecules take part in transportation (GLUT-4) across a membrane and also fight infection (antibodies).
RuBisCO (Ribulose bisphosphate carboxylase oxygenase), the enzyme in carbon fixation, is the most abundant protein present in the biosphere. Collagen is the most abundant protein present in animals.
Protein Structure
Protein is a heteropolymer of amino acids. A linear protein molecule has a sequence of amino acids, that are linked by a peptide bond. Proteins are also known as polypeptides.
Amino Acids
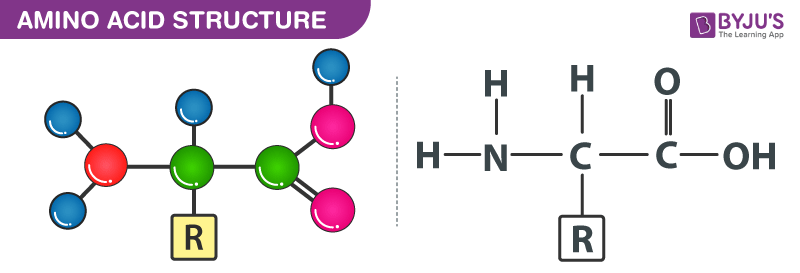
- Amino acids are organic compounds
- They are substituted methanes. The 𝜶- carbon has various substituents so they are also called 𝜶- amino acids
- The 𝜶- carbon has an amino group, carboxylic group, hydrogen and a variable R group
- Based on the nature of the R group, there are 20 amino acids present in proteins
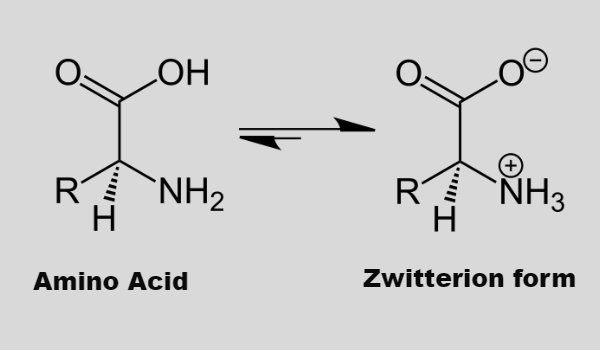
- Amino acids can exist in a zwitterionic form, which changes according to the pH of the solution to NH3+ (at low pH) or COO– (at high pH)
- Essential amino acids are required through diet. There are 9 essential amino acids: valine, leucine, isoleucine, phenylalanine, tryptophan, lysine, histidine, methionine and threonine
- Non-essential amino acids are formed inside the body and include glycine, alanine, arginine, aspartic acid, asparagine, cysteine, glutamine, glutamic acid, serine, proline and tyrosine
- Amino acids can be basic (lysine), acidic (glutamic acid) or neutral (valine) based on the R group present
- Tryptophan, tyrosine and phenylalanine have an aromatic side chain
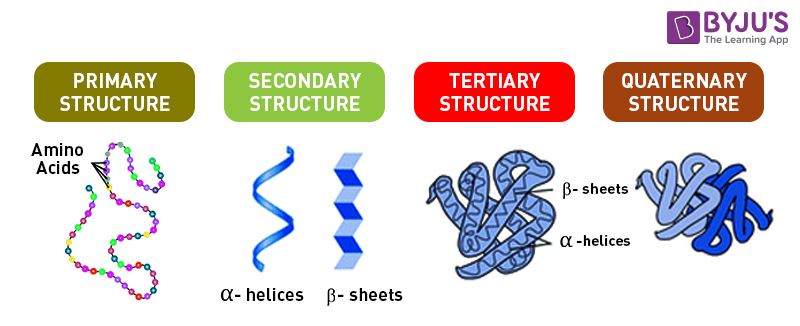
Primary Structure of protein is a long chain of amino acids in a specific sequence. The amino acid chain starts from N’ and ends with C’. amino acids are linked by C-N peptide bonds. The peptide bond is formed between the carboxyl (-COOH) of one amino acid and amino group (-NH2) of another amino acid. The process of peptide bond formation involves dehydration, one water molecule is released.
Secondary Structure of a protein has a helical structure. The long thread of amino acids is folded right-handed to form 𝜶-helix or are arranged as 𝛃-pleated sheets, which consist of 𝛃-strands, laterally joined by at least 2-3 hydrogen bonds.
Tertiary Structure of protein is highly folded. Protein chains are folded like a hollow woollen ball. It is a three-dimensional organisation, which is required for optimum activity.
Quaternary Structure is required when a protein is made up of more than one polypeptide chain or has subunits. These spheres of subunits or polypeptides may be arranged as linear strings or form a cube or plate. E.g. Haemoglobin consists of 4 subunits, 2 subunits each of 𝛼 and 𝛽 type.
Carbohydrates or Polysaccharides
Carbohydrates are polymers of monosaccharides or simple sugars. They are the main energy source of plants and animal cells. They have a general formula of [Cx(H2O)y]n.
Reducing sugars- All monosaccharides are reducing sugar. Some disaccharides are reducing, e.g. lactose, maltose, etc. They act as a reducing agent and reduce Tollen’s, Fehling or Benedict’s reagents. They have free aldehyde or ketone group (in a cyclic form hemiacetal or hemiketal group)
Non-reducing sugars- Sucrose (a disaccharide) is non-reducing as both the carbonyl groups are involved in the glycosidic bond formation. All the polysaccharides (cellulose, starch) are non-reducing
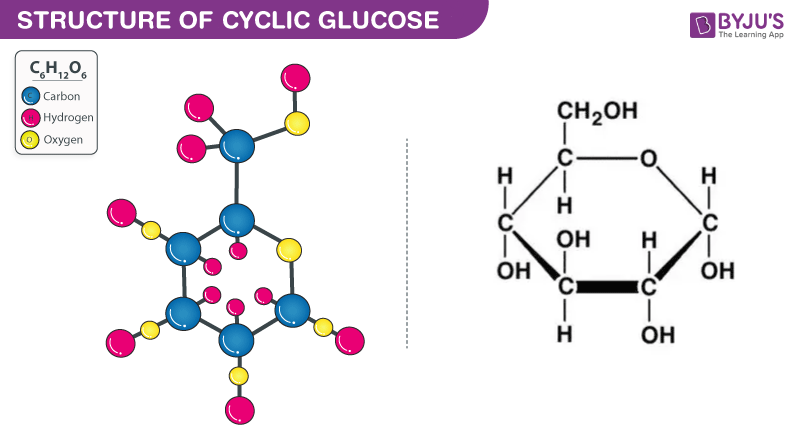
Monosaccharides- Glucose, Galactose, Fructose, ribose, etc. They are the building blocks of polysaccharides
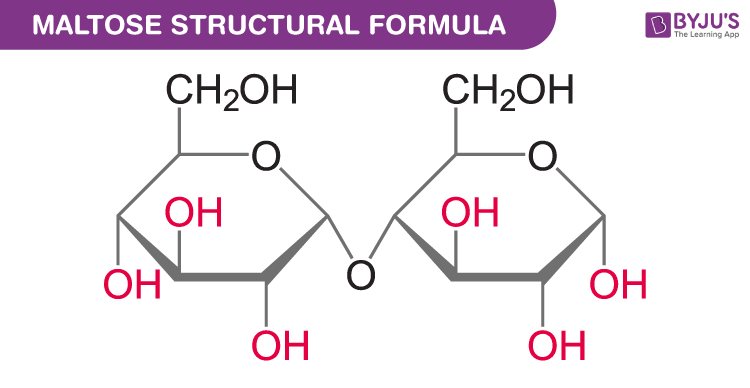
Disaccharides- They are the dimer of the same or different monosaccharides. The monosaccharides are linked by a glycosidic bond between two carbons of the monomers.
Maltose or malt sugar is made up of two 𝛼-D-glucose molecules linked by 1,4 linkage
Lactose is milk sugar and made up of 𝛽-D-galactose and 𝛽-D-glucose by 1,4 linkage
Sucrose is made up of glucose and fructose
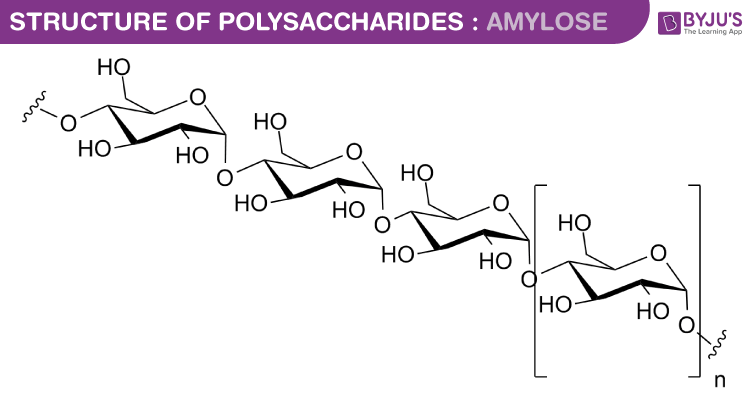
Structure and Function of Polysaccharides
They perform various functions and provide energy to the living cells. They constitute the cell wall stored as a food reserve. Starch, cellulose and glycogen are homopolymers of glucose. Heteropolymers are found in pectins, gums, etc.
Cellulose is a homopolymer of 𝛽-D-glucose molecules linked by 𝛽-1,4 glycosidic bonds. Cellulose is present in the cell wall of plants. Cotton fibres are cellulosic. Cellulose is a long-chain polymer and adjacent chains are linked by hydrogen bonds.
Starch is a major food reserve of plants. It is a polymer of glucose. It contains amylose and amylopectin.
- Amylose has 𝛼-1,4 linkage of D glucose. The chains are coiled to form a secondary helical structure, which traps I2 to form a blue colour complex
- In Amylopectin, linear chains are linked by 𝛼-1,4 glycosidic bonds and branches are formed by 𝛼-1,6 linkage
Glycogen is a food reserve for animals. It is similar to glycogen in structure, but it is more branched.
Inulin- polymer of fructose, present in many plants. It is found in roots and rhizomes.
Complex carbohydrates- They are polymer of substituted sugars such as amino sugars or sugar acids, e.g. N-acetylglucosamine, N-acetylmuramic acid.
- Chitin is a polymer of N-acetyl glucosamine. It is present in the exoskeleton of insects, cell wall of fungi, scales of fish, etc.
- Peptidoglycan (Murein) is a polymer of sugar and amino acids. Sugars present in peptidoglycan are the alternating unit of N-acetylglucosamine and N-acetylmuramic acid. The bacterial cell wall is made up of peptidoglycan.
Lipids
Fats, oil, wax, steroids, cholesterol are all lipids. They play an important role in metabolism. They are high energy-yielding compounds. Lipids are water-insoluble and found in membranes and also present as hormones and energy reserves.
- Fatty acids are carboxylic compounds having a long chain R group attached to it. The alkyl group could contain 1 C (methyl) to 19 carbons and can be saturated (contains only single bonds) or unsaturated (one or more C=C double bonds). Palmitic acid- 15 C R group, Arachidonic acid- 19 C R group
- Essential fatty acids are not formed in the body, e.g. linoleate, non-essential amino acids are produced in the body
- Monoglycerides, diglycerides and triglycerides are esters of fatty acids with glycerol and are known as fats. Oils have low m.p. and remain in liquid form in winters too
- Complex lipids contain more substituent in addition to fatty acids and alcohol. Phospholipids are present in the membrane, e.g. lecithin
- Steroids are present as hormones and have a tetracyclic structure. Cholesterol is the main steroid and is a precursor of many steroids hormones
Explore more: Ketogenesis
Nucleic Acids
Nucleic acids are found in all the living cells. Even a virus contains nucleic acids (DNA or RNA). DNA and RNA contain genetic information.
- Friedrich Miescher discovered nucleic acid and named it nuclein
- They are also known as polynucleotides, a polymer of nucleotide
Nucleotide
A nucleotide has three components: a nitrogenous base, a pentose sugar and a phosphate group
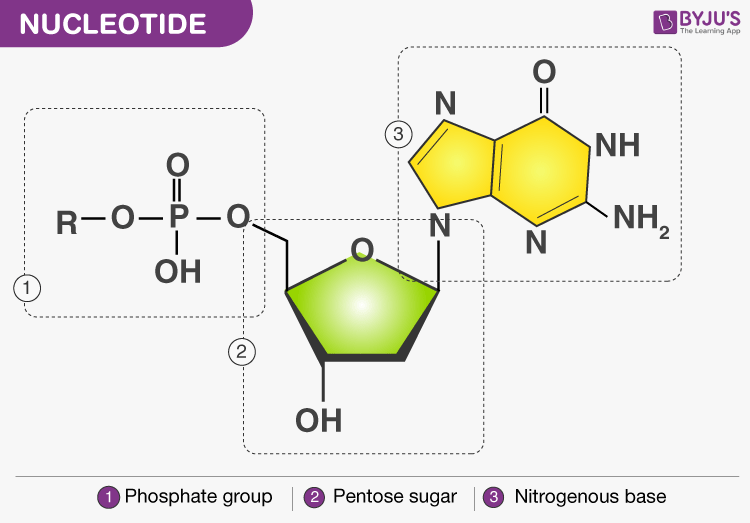
Nitrogenous base- There are two types of nitrogenous bases:
- Purine: Adenine and Guanine
- Pyrimidine: Thymine, Cytosine and Uracil
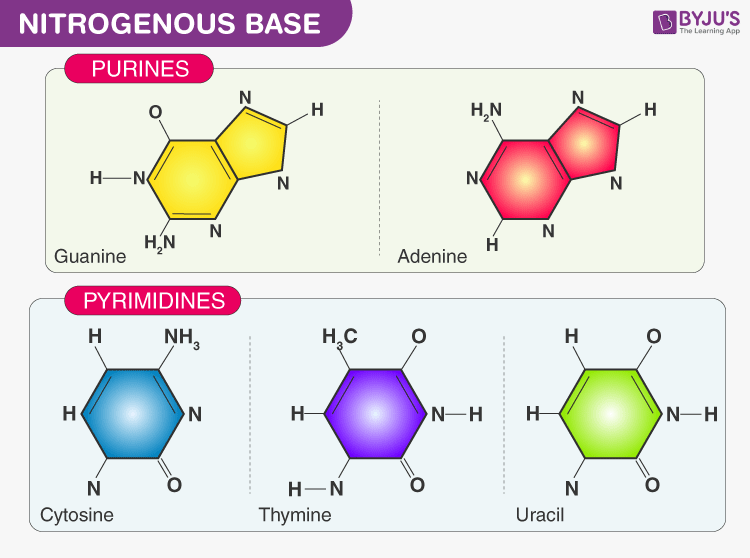
Sugar- DNA contains deoxyribose sugar and RNA contains ribose sugar molecules.
Nitrogenous bases attached to sugar are called Nucleosides.
Phosphate- Phosphate group is attached to sugar by the phospho-ester bond. Nucleosides with attached phosphates are called Nucleotides.
Nucleotides are joined by 3’- 5’ phosphodiester bonds to form polynucleotides. DNA and RNA are polynucleotides. The long chain of polynucleotides is folded further to form secondary and tertiary structures.
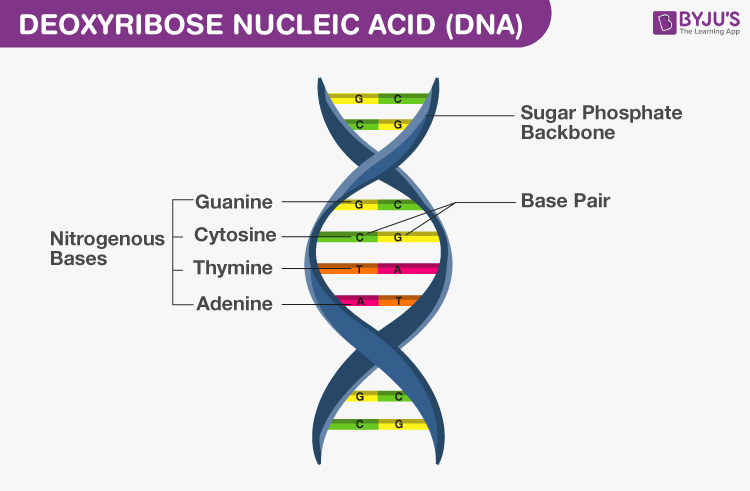
DNA
- Watson and Crick gave the double helix model
- DNA consists of antiparallel strands of polynucleotide chains, which are coiled in the right-hand direction
- The backbone of DNA is formed by sugar and phosphate and the nitrogenous bases are projected inside
- Nitrogenous bases of one strand are attached to the nitrogenous bases of the antiparallel strand
Adenine pairs with thymine by two hydrogen bonds (A=T)
Guanine (G) pairs with cytosine (C) by three hydrogen bond
- Each helical turn is composed of 10 base pairs with a pith of 34 Å. The distance between any two base pairs is 3.4 Å
- DNA is packaged in the form of nucleosomes and then further to chromatin fibres of chromosomes
To read more about DNA structure and inheritance click here
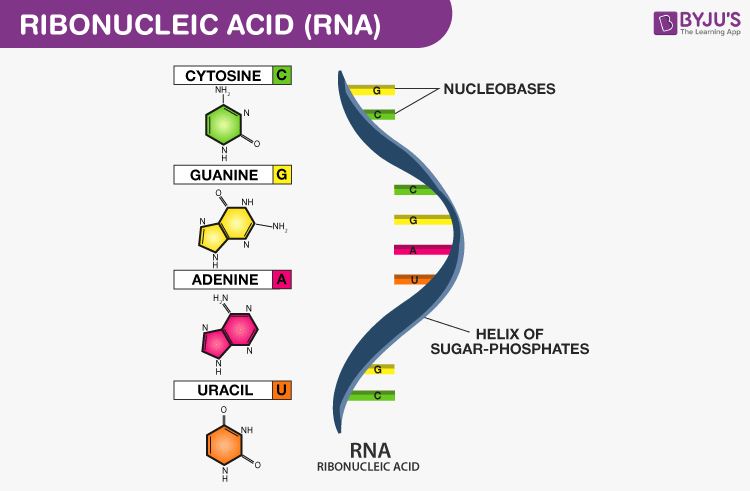
RNA
- RNA is a single-stranded polynucleotide chain
- RNA contains ribose sugar and uracil in place of thymine
- There are three types of RNA, mRNA, tRNA and rRNA
- mRNA provides the template for protein synthesis
- Ribozymes are RNA molecules. They act as an enzyme and catalyse biochemical reactions
Enzymes
Enzymes catalyse metabolic reactions in our body. Most of the enzymes are proteins, ribozymes are RNA, which act as an enzyme.
Enzymes have an active site where the specific substrate binds. A transient ES complex is formed, which is converted to EP complex and then the product is released with unchanged enzymes.
Temperature, pH and concentration of substrate regulate the enzyme activity. Enzyme activity is also regulated by binding of other substrates, e.g. competitive inhibitor, inhibit the reaction by binding at the active site. Malonate inhibits the activity of succinate dehydrogenase as it binds to the active site due to its similarity with the actual substrate, succinate.
Many enzymes have an allosteric regulation mechanism. In allosteric regulation, effector (inhibitor or activator) binds to a site other than the active site to bring about conformational changes and thereby affecting the activity of the enzyme.
Product may also act as an inhibitor, this type of regulation is called feedback regulation.
Enzymes get damaged by at the temperature above 40℃, but enzymes of thermophilic organisms remain stable at high temp also.
Also see: Biochemical Pathways
There are six major classes of enzymes:
- Dehydrogenases or oxidoreductases- One of the substrate gets oxidised and another reduced, e.g. oxidases, reductases, dehydrogenases
- Transferases- Catalyses the transfer of a group from one substrate to another, e.g. transaminase, transketolase, transaldolase
- Hydrolases- Hydrolysis of various bonds such as glycosidic, ester, peptide, etc, e.g. amylases, lipases, proteases, nucleases
- Lyases- Removal of a group by other than hydrolysis. A double bond is formed, e.g. aldolases, decarboxylases, fumarase, citrate synthase
- Isomerases- Formation of isomers (positional, geometrical or optical), e.g. isomerase, epimerase, mutase
- Ligases- joining of two compounds, i.e. formation of C-O, C-S, C-N, P-O bonds, e.g. synthetases, carboxylases
Cofactors: Cofactors are a non-protein part of the enzyme that is required for the activity of an enzyme. The protein part of the enzyme is known as apoenzyme. Catalytic activity is lost after the removal of cofactors. There are three types of cofactors:
- Prosthetic group- They are organic compounds tightly bound to the apoenzyme, e.g. haem is a prosthetic group of enzymes catalase and peroxidase
- Co-enzymes- They are organic compounds associated with the enzyme for a short period of time. Many vitamins act as a coenzyme catalysing various reactions, e.g. coenzymes NAD and NADP contain niacin
- Metal ions- Many metal ions work as cofactor. They get attached to the active site by forming a coordination bond and also form a coordination bond with the substrate, e.g. Zn metal acts as a cofactor of carboxypeptidase
Explore the next chapter for important points with regards to the NEET exam only at BYJU’S. Check the NEET Study Material for all the important concepts and related topics.
Also see:
Flashcards Of Biology For NEET Biomolecules
NEET Flashcards: Cell The Unit Of Life
NEET Flashcards: Cell Cycle And Cell Division
NEET Flashcards: Molecular Basis Of Inheritance
Frequently Asked Questions on Biomolecules
Is Biomolecules important for NEET?
Biomolecules is an important topic constituting the NEET syllabus. This chapter features in the important chapters for NEET that must be covered in order for students to perform exceedingly well. Year on, the weightage keeps varying, however, on an average 2-3 questions appear from this chapter. Hence, it is important and cannot be missed.
What is the role of biomolecules in diagnosis of diseases?
Biomolecules are vital in all life processes and activities. This includes disease development. Hence, the accurate detection of biomolecules is important in the diagnosis of diseases and in therapy.
What are essential fatty acids?
Recommended Video:
Biomolecules Class 12 Chemistry One Shot | Chapter 14 Chemistry Class 12 | NEET 2022 Chemistry Exam
very nice
Nice
Nice