CBSE Chemistry Practical Class 11 is an important part of the assessment for students. The marks obtained in CBSE Chemistry Class 11 Practical exams are added to the theory marks to prepare the final score of the students in the subject. Therefore, it’s crucial for students to go through the practical syllabus and activities mentioned in the Chemistry Lab Manual Class 11. It will help them in scoring high marks in the practical exams. Here, students will find the list of CBSE practicals and details on project works that they can perform and learn during the academic year.
CBSE Class 11 Chemistry Practical carries 30 marks in the exam. Therefore, students must perform Class 11 Chemistry Practicals effectively to score high marks in the subject. The Chemistry Practical for Class 11 consists of volumetric analysis for 8 marks, salt analysis for 8 marks, content-based experiments for 6 marks, project work, class record and viva for 4 marks, respectively. All these details are mentioned in the Chemistry Lab Manual Class 11. To download the CBSE Syllabus for Class 11 Chemistry Practicals, click on the link below.
Download CBSE Class 11 Chemistry Practical Syllabus PDF 2023-24
CBSE Syllabus for Class 11 Chemistry Practical Evaluation Scheme
Below you will find the CBSE Class 11 Chemistry Practical evaluation scheme for students.
| Topics | Marks |
| Volumetric Analysis | 8 |
| Salt Analysis | 8 |
| Content Based Experiment | 6 |
| Project Work | 4 |
| Class record and viva | 4 |
| Total | 30 |
CBSE Class 11 Chemistry Practical Syllabus 2023-24
Below you will find the CBSE Class 11 Chemistry practical syllabus for students.
CBSE Class 11 Chemistry Practical Syllabus: List of Experiments
Chemistry is an interesting subject as it gives knowledge about the behaviour of matter, composition, properties, etc. The Class 11 Chemistry Practical Exam Syllabus is designed by the CBSE Board in such a way that it evaluates a student’s expertise thoroughly. Students must go through these practicals and Chemistry Lab Manual Class 11 to prepare more efficiently for the final exam.
| CBSE Class 11 Chemistry Practical |
| A. Basic Laboratory Techniques |
| 1. Cutting glass tube and glass rod |
| 2. Bending a glass tube |
| 3. Drawing out a glass jet |
| 4. Boring a cork |
| B. Purification and Criteria of Purity |
| 1. Determination of the melting point of a solid organic compound. |
| 2.Determination of the boiling point of a liquid organic compound. |
| 3. Crystallization of impure sample of any one of the following: Alum, Copper Sulphate, Benzoic Acid. |
| C. Experiments based on pH |
| a) Any one of the following experiments:
• Determination of pH of some solutions obtained from fruit juices, solution of known and varied concentrations of acids, bases and salts using pH paper or universal indicator. • Comparing the pH of solutions of strong and weak acids of same concentration. Study the pH change in the titration of a strong base using universal indicator. |
| b) Study the pH change by common-ion in case of weak acids and weak bases. |
| D. Chemical Equilibrium |
| One of the following experiments: a) Study the shift in equilibrium between ferric ions and thiocyanate ions by increasing/decreasing the concentration of either of the ions |
| b) Study the shift in equilibrium between [Co(H2O)6]2+ and chloride ions by changing the concentration of either of the ions. |
| E. Quantitative Estimation |
| i. Using a mechanical balance/electronic balance. |
| ii. Preparation of standard solution of Oxalic acid. |
| iii. Determination of strength of a given solution of Sodium hydroxide by titrating it against standard solution of Oxalic acid. |
| iv. Preparation of standard solution of Sodium carbonate. |
| v. Determination of strength of a given solution of hydrochloric acid by titrating it against standard Sodium Carbonate solution. |
| F. Qualitative Analysis |
| a) Determination of one anion and one cation in a given salt |
| Cations – Pb2+, Cu2+, Al3+, Fe3+, Mn2+, Ni2+, Zn2+, Co2+, Ca2+, Sr2+, Ba2+, Mg2+, NH4+
Anions – (CO3)2‒, S2‒, NO2–, SO32‒, SO42‒, NO3‒, Cl‒, Br‒, I‒, PO43‒, C2O42‒, CH3COO‒ |
| b) Detection of -Nitrogen, Sulphur, Chlorine in organic compounds. |
Also Check: CBSE Class 11 Chemistry Viva Questions
List of Suggested Projects for CBSE Class 11 Chemistry Practical
Scientific investigations involving laboratory testing and collecting information from other sources.
A few suggested projects are listed below:
• Checking the bacterial contamination in drinking water by testing sulphide ion
• Study of the methods of purification of water
• Testing the hardness, presence of Iron, Fluoride, Chloride, etc., depending upon the regional variation in drinking water and study of causes of presence of these ions above permissible limit (if any).
• Investigation of the foaming capacity of different washing soaps and the effect of addition of Sodium carbonate on it.
• Study the acidity of different samples of tea leaves.
• Determination of the rate of evaporation of different liquids Study the effect of acids and bases on the tensile strength of fibres.
• Study of acidity of fruit and vegetable juices.
CBSE Chemistry Lab Manual Class 11
The list of experiments mentioned in the Chemistry Lab Manual Class 11 is as follows:
EXPERIMENT-1
PREPARATION OF STANDARD SOLUTION OF OXALIC ACID:
AIM:

THEORY:


MATERIAL REQUIRED:

PROCEDURE:


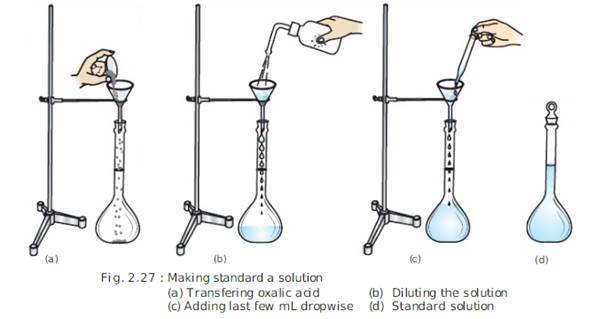
PRECAUTIONS:

EXPERIMENT-2
PURIFICATION OF SAMPLE OF A COMPOUND BY CRYSTALLISATION:
AIM:

THEORY:


MATERIAL REQUIRED:
PROCEDURE:

PRECAUTIONS:

EXPERIMENT-3
DETERMINATION OF THE MELTING POINT OF AN ORGANIC COMPOUND:
AIM:
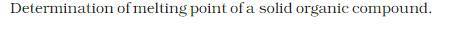
THEORY:

MATERIAL REQUIRED:

PROCEDURE:





PRECAUTIONS:

EXPERIMENT-4
DETERMINATION OF BOILING POINT OF AN ORGANIC COMPOUND:
AIM:
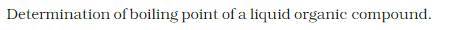
THEORY:

MATERIAL REQUIRED:
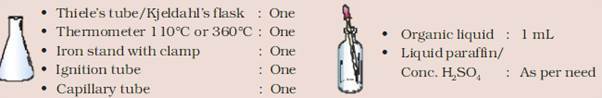
PROCEDURE:

PRECAUTIONS:

EXPERIMENT-5
STUDY OF SHIFT IN EQUILIBRIUM IN THE REACTION OF FERRIC IONS AND THIOCYANATE IONS:
AIM:

THEORY:
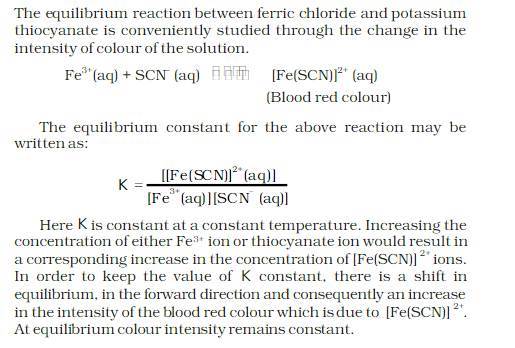
MATERIAL REQUIRED:

PROCEDURE:

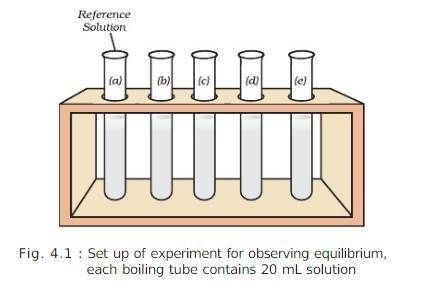

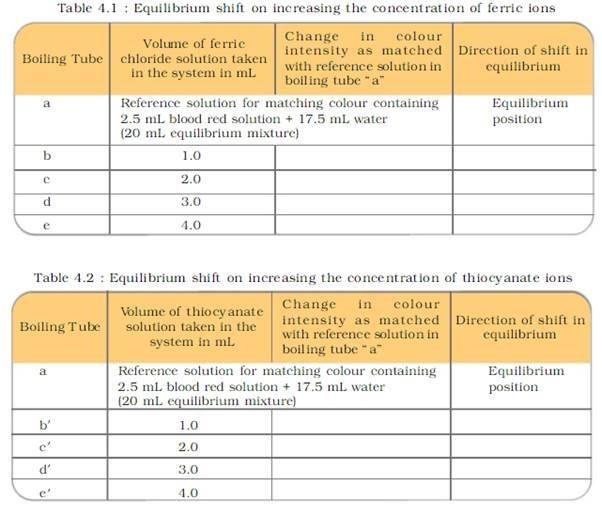
PRECAUTIONS:

EXPERIMENT-6
TO DETERMINE THE pH OF SOME FRUIT JUICES:
AIM:
THEORY:


MATERIAL REQUIRED:
PROCEDURE:


RESULT:

PRECAUTIONS:

EXPERIMENT-7
DETERMINATION OF THE CONCENTRATION (STRENGTH) OF A GIVEN SODIUM HYDROXIDE SOLUTION BY TITRATING IT AGAINST A STANDARD SOLUTION OF OXALIC ACID:
AIM:

THEORY:
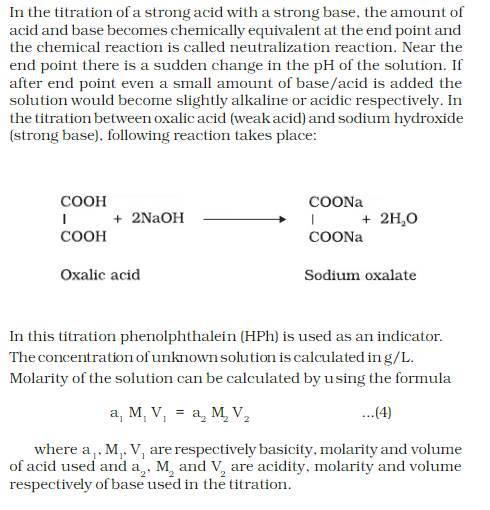
MATERIAL REQUIRED:

PROCEDURE:




CALCULATION:

RESULT:

PRECAUTIONS:

Stay tuned to BYJU’S to get the latest notifications on CBSE along with the CBSE syllabus, sample papers, marking scheme and more.
Osm