Analysis of metallic elements in compounds or samples is an integral part of chemical research. The purpose of this experiment is to learn the techniques to separate and identify some common cations and to understand the principles for the equilibria of precipitation and complex formation. The systematic analysis of cations is an integral part of the salt analysis (or systematic qualitative analysis).
Table of Contents
- Aim
- Theory
- Materials Required
- Procedure
- Observation and Inference
- Results
- Precautions
- Frequently Asked Questions on Systematic Analysis of Cations
Aim
To identify the cationic radicals present in an inorganic mixture of salts by performing various tests.
| Also Read: Systematic Analysis of Cations Viva Questions |
Theory
Qualitative analysis is the systematic approach that involves precipitation reaction to remove cations sequentially from a mixture. The behaviour of the cations toward a set of common test reagents differs from one cation to another and furnishes the basis for their separation.
The qualitative analysis of an inorganic mixture is started by first carrying out some preliminary tests. The Preliminary tests for cations are
- Physical examination
- Charcoal cavity test
- Boraxbead test
- Flame test
These tests do not give conclusive evidence yet they provide some information about the ions present in the mixture.
Qualitative analysis of cations usually consists of three stages.
- First based on different solubility properties the cations are separated into 5 groups through the successive addition of selective precipitating reagents.
- Second, within each group precipitated cations are separated through selective dissolution processes.
- Third, the presence of each cation is verified through different identification tests.
The cations are classified into the following 5 groups.
1. Group I Cations (Ag+, Hg22+ and Pb2+ – insoluble chlorides):
Among the common metallic cations only three cations form insoluble chlorides with hydrochloric acid. When 6M of HCl is added to the solution, white precipitates of AgCl, Hg2Cl2 and PbCl2 are formed. Other metallic cations remain in solution.
2. Group II Cations (Hg2+, Pb2+, Cu2+, Bi3+, Cd2+, As3+, Sb3+ and Sn4+ – insoluble sulphides in acidic medium):
After the insoluble chlorides are isolated, the pH of the solution is adjusted to 0.5 and then H2S is added. Since the concentration of sulphide ion (s2-) is very low at low pH, only those metallic sulphided having very low Ksp values will precipitate. Cations with larger Ksp values for their sulphides remain in solution.
3. Group III Cations (Al3+. Fe3+, Co2+, Ni2+, Cr3+, Zn2+ and Mn2+ – insoluble sulphides or hydroxides in alkaline medium):
After isolating the insoluble sulphides in acidic medium, the solution is made basic and the metallic sulphides having larger Ksp values such as ZnS, NiS, CoS and MnS precipitate. Moreover, since the solution is basic Al3+, Fe3+ and Cr3+ form insoluble hydroxides and are also separated from the solution.
4. Group IV Cations (Ca2+, Sr2+ and Ba2+ – carbonate precipitates):
These three metallic cations all belong to Group IIA in the periodic table of elements and therefore their chemical properties are very similar. They form soluble chlorides and sulphides and hence are separable from groups 1, 2 and 3 cations. However, their carbonates precipitate in a mixture of ammonium carbonate, ammonium chloride or ammonia solution.
5. Group V Cations (Mg2+, Na+, K+ and NH4+):
None of the cations in this group form precipitates in the separation processes of group 1-4 cations and thus remain in the final solution.
The flowchart for separating the five groups of cations is given below.
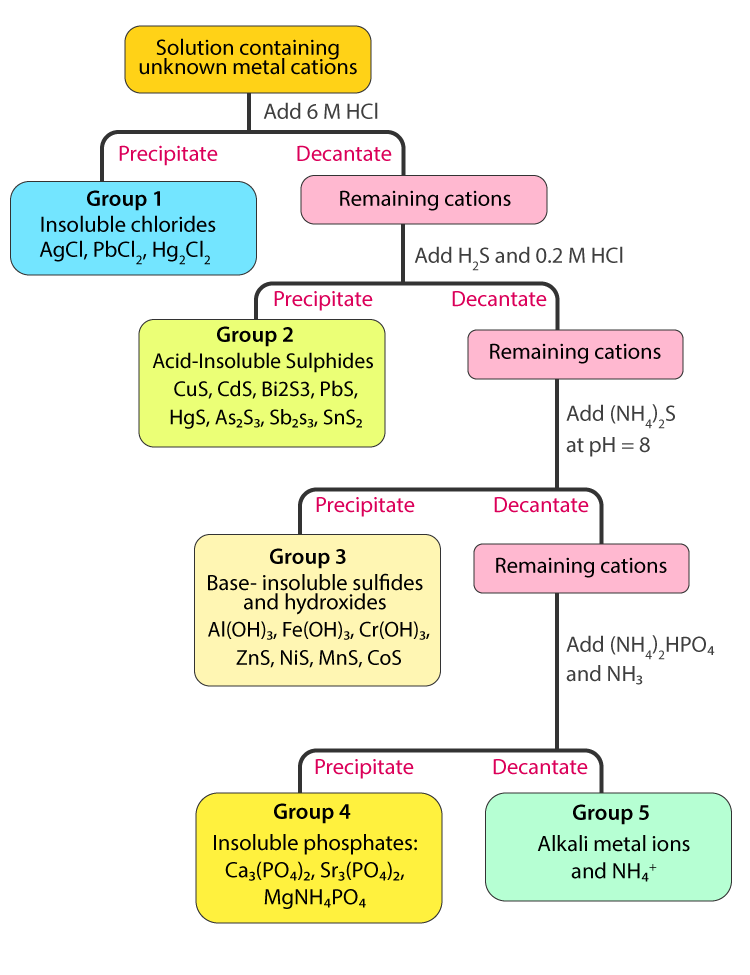
Classification of Cations
The list of cations comprising the seven groups and the group reagent used for precipitating them out from the solution is tabulated below.
| Group | Cations | Group Reagents | |
| Zero | NH4+, K+ | Tested using the mixture. | |
| I | Ag+, Hg22+, Pb2+ | HCl | |
| II | A | Hg2+, Pb2+, Cu2+, Bi3+, Cd2+ | |
| B | As3+, Sb3+, Sn2+ | H2S in the presence of HCl | |
| III | Al3+, Cr3+, Fe3+ | NH4Cl + NH4OH | |
| IV | Zn2+, Mn2+, Co2+, Ni2+ | H2S in presence of NH4Cl and NH4OH | |
| V | Ba2+, Ca2+, Sr2+ | NH4Cl + NH4OH + (NH4)2CO3 | |
| VI | Mg2+, K+ | ||
Materials Required
- Test tubes
- Boiling tubes
- Test tube holder
- Test tube stand
- Corks
- Filter paper
- Delivery tube
- Reagents
- Measuring cylinder
Procedure
(i) Preliminary Test of Cations
| Colour | Inference |
| Blue or bluish green | Copper or Nickel salts |
| Light green | Ferrous salts |
| Dark green | Chromium salts |
| Dark brown | Ferric salts |
| Light pink or Flesh colour | Manganese salts |
| White | Absence of Cu, Ni, Fe, Mn and Co salts |
(ii) Charcoal Cavity Test
In this test the cations is converted into the metal carbonate in a charcoal cavity which decomposes on heating to metal oxide or even to the metallic state. The cation present can be detected from the colour of the bead or residue left in the cavity or deposit formed outside the cavity called incrustation.
ZnSO4 + Na2CO3 → ZnCO3 + Na2SO4
ZnCO3 → ZnO (yellow when hot) + CO2
CuSO4 + Na2CO3 → CuCO3 + Na2SO4
CuCO3 → CuO + CO2
CuO + C → Cu (reddish) + CO
CdCl2 + Na2CO3 → CdCO3 + 2NaCl
CdCO3 → CdO (reddish brown) + CO2
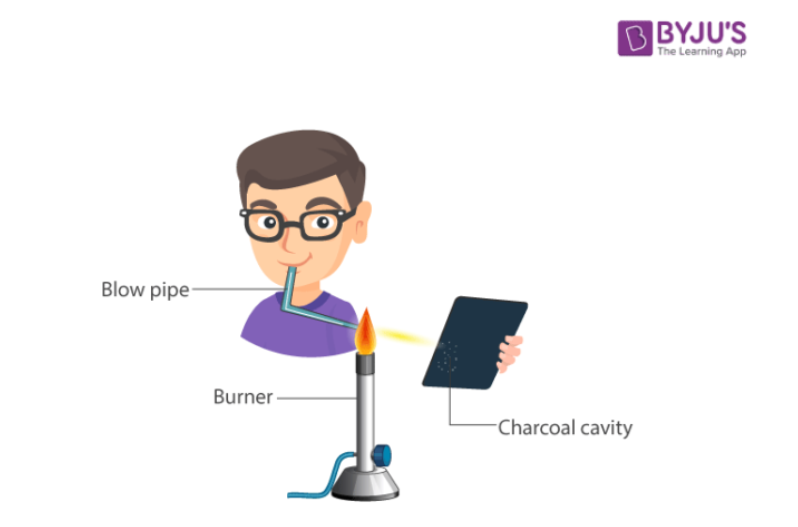
Procedure for Charcoal Cavity Test
Make a small cavity in charcoal block with the help of a borer. Mix the given mixture with sodium carbonate or fusion mixture, moisten it with a drop of water, heat it in the charcoal cavity in the reducing flame with the help of a mouth blowpipe and observe the colour of the residue left.
(iii) Borax Bead Test
This test is used for the detection of cations in coloured mixtures which may contain copper, nickel, iron or manganese. The borax bead reacts with these metal oxides to form metabolites which have characteristic colour. Some metaborates when heated in reducing flame form lower metaborates or even metals thus causing a change in the colour of bead.
Na2B4O7.10H2O → Na2B4O7(White porous mass) → NaBO2 + B2O3
B2O3 + CuSO4 → Cu(BO2)2(blue green cupric metaborate) + SO3↑
2Cu(BO2)2 + C → 2CuBO2(colourless coprous metaborate) + B2O3 + CO↑
2CuBO2 + C → 2Cu (red) + B2O3 + CO↑
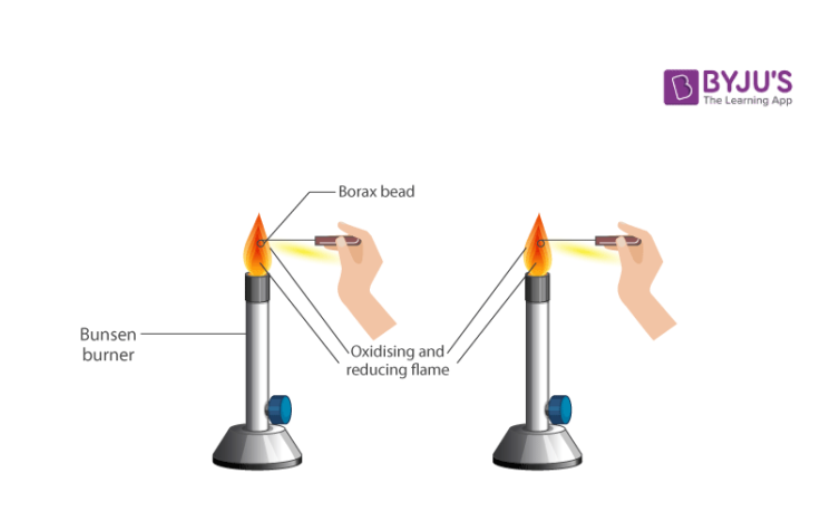
Procedure for Borax Bead Test
- Make a small loop at the end of the platinum wire.
- Heat the loop in a Bunsen flame until it is red hot.
- Dip it into powdered borax placed on a watch glass and heat again. The borax will first swell up and will fuse giving a colourless, transparent glass like bead.
- Touch the hot bead with conc.HCl and touch it with the given mixture or salt. Heat it in an oxidising (non-luminous) and reducing (luminous) flame. Observe the colour of the bead and draw inferences.
(iv) Flame Test
Certain cations like fifth group radicals Ba2+, Sr2+ and Ca2+ in the form of their chlorides impart characteristic colours to the non-luminous flame. The chlorides of these cations are thermally ionised. The ions so formed absorb heat energy and get excited. The extra energy is released in the form of light of the characteristic colour in the visible part of spectrum. Since different metal ions emit light energy of different wavelengths, the colours imparted by the different cations to the flame are also different.
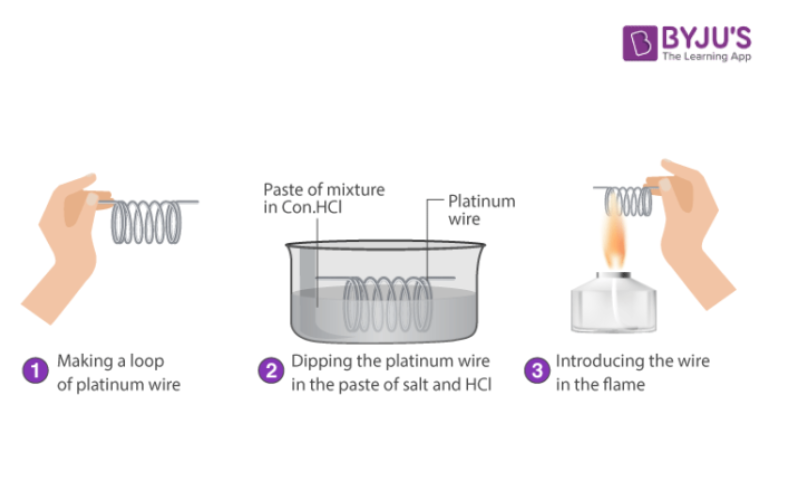
Procedure for Flame Test
- Take a platinum wire and dip it in conc.HCl taken on a watch glass and heat it strongly in the flame. Repeat this process until the wire does not impart any colour to the flame.
- Dip platinum wire in con.HCl and then touch it with a given salt and heat it in the flame of the Bunsen burner. Observe the colour of the flame and draw inferences.
Observation and Inference
(i) Charcoal Cavity Test
| S.No | Observation | Inference |
| 1 | Shining metallic bead with yellow incrustation soft and mark paper. | Pb2+(Lead) |
| 2 | Shining metallic bead does not mark paper. | Ag+(Silver) |
| 3 | Brittle bead with yellow or brown incrustation. | Bi3+(Bismuth) |
| 4 | White incrustation with white fumes having garlic odour. | As3+(Arsenic) |
| 5 | Brown residue and brown incrustation. | Cd2+(Cadmium) |
| 6 | Red residue without incrustation. | Cu2+(Copper) |
| 7 | Yellow residue and yellow incrustation when hot and white in cold. | Zn2+(Zinc) |
| 8 | White residue | May be Al3+, Ca2+, Ba2+ or Mg2+ |
| 9 | Black residue without incrustation. | Fe3+, Ni3+, Mn2+ |
| 10 | No bead, white liquid globule and smoke. | Hg (Mercury) |
(ii) Borax Bead Test
| S.No | Colour of the Bead in Oxidising Flame | Colour of the Bead in Reducing Flame | Inference | ||
| Hot | Cold | Hot | Cold | ||
| 1 | Green | Blue | Reddish or Colourless | Reddish or Colourless | Copper |
| 2 | Yellow | Yellow | Green | Green | Iron |
| 3 | Brown | Brown | Grey or Black | Grey or Black | Nickel |
| 4 | Pink | Pink | Colourless | Colourless | Manganese |
(iii) Flame Test
| S.No | Colour of the Flame | Inference | |
| With Naked Eye | Through Blue Glass | ||
| 1 | Golden Yellow | Nil | Sodium (Na+) |
| 2 | Violet | Pink | Potassium (K+) |
| 3 | Crimson red | Purple or crimson | Strontium (Sr2+) |
| 4 | Brick red | Light yellow | Calcium (Ca2+) |
| 5 | Light green | Bluish green | Barium (Ba2+) |
| 6 | Bluish green or blue | Bluish green or blue | Copper (Cu2+) |
| 7 | Flashes of green | Not characteristic | Zinc (Zn2+) or Manganese (Mn2+) |
Results
The given salt contains ________ (NH4+, K+, Ag+, Hg22+, Pb2+, Hg2+, Cu2+, Bi3+, Cd2+, As3+, Sb3+, Sn4+, Al3+, Fe3+, Co2+, Ni2+, Cr3+, Zn2+, Mn2+, Ca2+, Sr2+, Ba2+, Mg2+, Na+) cation.
Precautions
- Read the label carefully on the bottle before using any reagent or chemicals. Never use a reagent that is unlabeled.
- Always use an apron, an eye protector and hand gloves in the chemical laboratory.
- In smelling chemicals or vapours, be careful. Always gently fan the vapours to your nose
- Do not unnecessarily mix chemicals with reagents. Do not taste chemicals at all.
- For dilution, always pour acid into water. Never add acid to water.
- Never add or throw sodium metal into the sink or dustbin.
- Be careful as the test tube is heated. When heating or adding a reagent, the test tube should never point to yourself or your neighbours.
- For dilution, always pour acid into water. Never add acid to water.
- Keep clean your working environment. Never throw in the sink any papers and glass. For this purpose, always use dustbin.
- Be careful with explosive compounds, flammable substances, toxic gasses, electrical appliances, glass products, flames and hot substances.
- Always use the minimum quantity of reagents. The use of excessive reagents not only leads to chemicals being wasted, but also causes environmental damage.
- Always wash your hands after the laboratory work has been completed.
Frequently Asked Questions on Systematic Analysis of Cations
Name the group 1 cation which forms white precipitate.
Pb2+.
Name the group 2 cation which forms chocolate brown precipitate.
Cu2+.
Name the group 3 cation which produces a deep red colour.
Fe3+
Name the group 4 cation which obtains a black precipitate.
Ni2+.
Name the group 4 cation which obtains a white precipitate.
Sr2+
