Everything tries to achieve minimum energy to remain stable. In a chemical reaction, reactants undergo changes to lower the energy and the reaction proceeds until a low energy state is reached. In a state of chemical equilibrium, the concentration of reactants and products doesn’t change with time and there is no observable change in the system. In a dynamic equilibrium state, the rate of the forward reaction is equal to the rate of backward reaction.
Download the Complete Guide to NEET UG Prep
Download Now
1. Find the pH of a solution when 0.01 M HCl and 0.1 M NaOH are mixed in equal volumes
(a) 12.65
(b) 1.04
(c) 7.0
(d) 2.0
Answer: (a)
2. Which of the following aqueous solution will be the best conductor of electricity?
(a) NH3
(b) CH3COOH
(c) HCl
(d) C6H12O6
Answer: (c)
3. In 0.10 M aqueous solution of pyridine (C5H5N), find the percentage of pyridine that forms pyridinium ion (C5H5N+H) (Kb for C5H5N = 1.7 x 10-9 )
(a) 1.6%
(b) 0.77%
(c) 0.0060%
(d) 0.013%
Answer: (d)
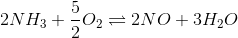
4. Find the equilibrium constant of the reaction
If the equilibrium constant for the following reactions are given
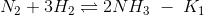
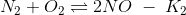
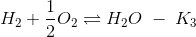
(a) K2K3/K1
(b) K1K33/K2
(c) K2K33/K1
(d) K23K3/K1
Answer: (c)
5. Highest pH will be recorded for which of the following solutions if they are equimolar
(a) AlCl3
(b) BaCl2
(c) BeCl2
(d) LiCl
Answer: (b)
6. The equilibrium constant is 278 for the reaction
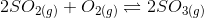
at the same temperature, what will be the equilibrium constant for the following reaction?
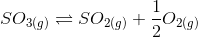
(a) 6 x 10-2
(b) 1.8 x 10-3
(c) 1.3 x 10-5
(d) 3.6 x 10-5
Answer: (a)
7. What will be the pH of a buffer solution having an equal concentration of B– and HB (Kb = 10-10 for B–)
(a) 7
(b) 4
(c) 10
(d) 6
Answer: (b)
8. Find the increase in equilibrium concentration of Fe3+ ions if OH– ions concentration decreases to 1/4th in the following reaction
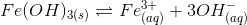
(a) 8 times
(b) 16 times
(c) 4 times
(d) 64 times
Answer: (d)
9. On increasing the concentration of reactants in a reversible reaction, then equilibrium constant will
(a) depend on the concentration
(b) increase
(c) unchanged
(d) decrease
Answer: (c)
10. Find the conjugate acid of NH2–
(a) NH3
(b) NH4OH
(c) NH4+
(d) NH2–
Answer: (a)
Recommended Videos
Introduction to Chemical Equilibrium

Equilibrium – Top 10 Questions

Chemical Equilibrium – Le Chatelier’s Principle

it is very useful