Everything around us is made up of atoms. Atom is the smallest unit of matter and has all the chemical properties of a matter. Atom has a positively charged nucleus, which contains positively charged particles known as protons and neutral particles called neutrons. The nucleus is surrounded by negatively charged electrons. In a neutral atom, the number of protons is equal to the number of electrons. An atomic number of an element is directly related to its chemical and physical properties.
Download Complete Chapter Notes of Structure of Atom
Download Now
1. How many orbitals can have the following set of quantum numbers, n = 3, l = 1, m1 = 0 ?
(a) 3
(b) 1
(c) 4
(d) 2
Answer: (b)
2. Electronic configuration of the outer shell of the element Gd with atomic number 64 is
(a) 4f4 5d5 6s1
(b) 4f3 5d5 6s2
(c) 4f5 5d4 6s1
(d) 4f7 5d1 6s2
Answer: (d)
3. Maximum number of electrons in a subshell can be
(a) 4l + 2
(b) 4l – 2
(c) 2n2
(d) 2l + 1
Answer: (a)
4. The orientation of atomic orbitals depends on their
(a) spin quantum number
(b) magnetic quantum number
(c) azimuthal quantum number
(d) principal quantum number
Answer: (b)
5. A gas X has Cp and Cv ratio as 1.4, at NTP 11.2 L of gas X will contain_______ number of atoms
(a) 1.2 × 1023
(b) 3.01 × 1023
(c) 2.01 × 1023
(d) 6.02 × 1023
Answer: (d)
6. Number of unpaired electrons in N2+
(a) 3
(b) 1
(c) 2
(d) 0
Answer: (b)
7. The excitation energy of a hydrogen atom from its ground state to its third excited state is
(a) 12.75 eV
(b) 0.85 eV
(c) 10.2 eV
(d) 12.1 eV
Answer: (a)
8. 3p orbital has _____ radial nodes
(a) three
(b) two
(c) one
(d) none
Answer: (c)
9. A 0.66 kg ball is moving with a speed of 100 m/s. Find its wavelength
(a) 6.6 × 10-34 m
(b) 6.6 × 10-32 m
(c) 1.0 × 10-32 m
(d) 1.0 × 10-35 m
Answer: (d)
10. Orbital angular momentum of p electrons is
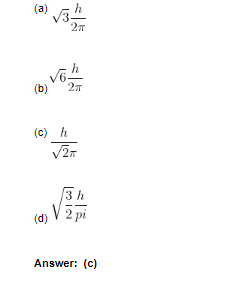
Recommended Video:
Atomic Structure – MCQs | Class 11 Chemistry | Rapid Fire Tuesday Quiz | NEET 2023

Nice
Superb