According to the CBSE Syllabus 2023-24, this chapter has been removed from NCERT Class 8 Science textbook.
Introduction
Everything around us is categorized into either metals or non-metals. Hence, it is important to know what non-metals and metals are and how to distinguish them. Class 8 Science Chapter 4 discusses the various physical and chemical properties of metals and non-metals. To help you with it, here we list a few physical and chemical properties of metals and non-metals.
For more information on Metal and Non-Metal Introduction, watch the below video

Metals
- Metals are described as chemical elements that readily lose valence electrons to form positive ions (cations).
Examples: Aluminium, copper, iron, tin and gold. - Around 90 of the total 118 elements are metals.
To know more about Metals, visit here.
Physical Properties
Physical Properties of Non-Metals
- Occur as solids, liquids, and gases at room temperature
- Brittle
- Non-malleable
- Non-ductile
- Non-sonorous
- Bad conductors of heat and electricity
Exceptions in Physical Properties
- Alkali metals (Na, K, Li) can be cut using a knife.
- Mercury is a liquid metal.
- Lead and mercury are poor conductors of heat.
- Mercury expands significantly for the slightest change in temperature.
- Gallium and caesium have a very low melting point
- Iodine is non-metal, but it has lustre.
- Graphite conducts electricity.
- Diamond conducts heat and has a very high melting point.
Physical Properties of Metals
● Hard and have a high tensile strength
● Solids at room temperature, except mercury, which is liquid at room temperature.
● Sonorous
● Good conductors of heat and electricity
● Malleable, i.e., can be beaten into thin sheets
● Ductile, i.e., can be drawn into thin wires
● High melting and boiling points (except Cesium (Cs) and Gallium (Ga))
● Dense (except alkali metals). Osmium – highest density, and lithium – least density
● Lustrous
● Silver-grey in colour (except gold and copper)
Non-Metals
Non-metals are those elements which do not exhibit the properties of metals.
Examples: Carbon, Boron, etc.
To know more about Non-Metals, visit here.
For more information on Physical Properties of Metals and Non-Metals, watch the below video:

To know more about Properties of Metals and Non-Metals, visit here.
Chemical Properties of Metals
● Alkali metals (Li, Na, K, etc) react vigorously with water and oxygen or air.
● Mg reacts with hot water.
● Al, Fe, and Zn react with steam.
● Cu, Ag, Pt, and Au do not react with water or dilute acids.
Chemical Properties
Displacement Reactions
A more reactive element displaces a less reactive element from its compound or solution.
i) Zn(s)+CuSO4(aq)→ZnSO4(aq)+Cu(s)
ii) 2Al(s)+Fe2O3(molten)→Al2O3(s)+2Fe(molten)
Metals Reaction with Oxygen (Burnt in Air)
Metal + Oxygen → Metal oxide (basic)
● Na and K are kept immersed in kerosene oil as they react vigorously with air and catch fire.
4K(s)+O2(g)→2K2O(s) (vigorous reaction)
● Mg, Al, Zn, and Pb react slowly with air and form a protective layer that prevents corrosion.
2Mg(s)+O2(g)→2MgO(s) (Mg burns with a white dazzling light)
4Al(s)+3O2(g)→2Al2O3(s)
● Silver, platinum, and gold don’t burn or react with air.
Basic Oxides of Metals
Some metallic oxides get dissolved in water and form alkalis. Their aqueous solution turns red litmus blue.
Na2O(s)+H2O(l)→2NaOH(aq)
K2O(s)+H2O(l)→2KOH(aq)
Amphoteric Oxides of Metals
Amphoteric oxides are metal oxides which react with both acids as well as bases to form salt and water.
For example – Al2O3, ZnO, PbO, SnO
Al2O3(s)+6HCl(aq)→2AlCl3(aq)+3H2O(l)
Al2O3(s)+2NaOH(aq)→2NaAlO2(aq)+H2O(l)
ZnO(s)+2HCl(aq)→ZnCl2(aq)+H2O(l)
ZnO(s)+2NaOH(aq)→Na2ZnO2(aq)+H2O(l)
For more information on Chemical Properties of Metal and Non-Metals, watch the below video

To know more about Chemical Properties of Metals, visit here.
Reactivity Series
The below table illustrates the reactivity of metals from high order to low order.
| Symbol | Element |
| K | Potassium ( Highly Active Metal) |
| Ba | Barium |
| Ca | Calcium |
| Na | Sodium |
| Mg | Magnesium |
| Al | Aluminium |
| Zn | Zinc |
| Fe | Iron |
| Ni | Nickel |
| Sn | Tin |
| Pb | Lead |
| H | Hydrogen |
| Cu | Copper |
| Hg | Mercury |
| Ag | Silver |
| Au | Gold |
| Pt | Platinum |
To know more about Reactivity Series, visit here.
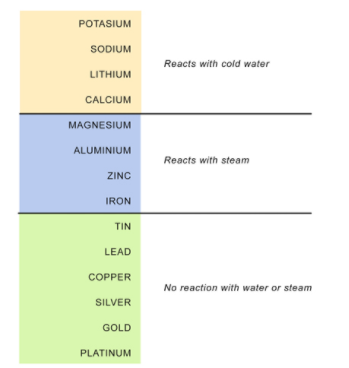
Reaction of Metals with Water/Steam
Metal+Water→Metal hydroxide or Metal oxide+Hydrogen
2Na+2H2O(cold)→2NaOH+H2+heat
Ca+2H2O(cold)→Ca(OH)2+H2
Mg+2H2O(hot)→Mg(OH)2+H2
2Al+3H2O(steam)→Al2O3+3H2
Zn+H2O(steam)→ZnO+H2
Reaction of Metals with Acid
- Metals, which are more reactive than hydrogen, displace hydrogen from its dilute acids and produce respective metal salts and hydrogen gas.
- Example: Metal+dilute acid→Salt+Hydrogen gas
2Na(s)+2HCl(dilute)→2NaCl(aq)+H2(g)
2K(s)+H2SO4(dilute)→K2SO4(aq)+H2(g) - Metals, which are less reactive than hydrogen, cannot displace hydrogen from their acids, and hence no reaction takes place.
How Do Metals React with Solution of Other Metal Salts
- High-reactive metal displaces the low-reactive metal from its salt solution.
Metal A+Salt of metal B→Salt of metal A+Metal B
Fe(s)+CuSO4(aq)→FeSO4(aq)+Cu(s)
Reaction of Metals with Bases
Base+metal→salt+hydrogen
2NaOH(aq)+Zn(s)→Na2ZnO2(aq)+H2(g), when zinc reacts with aqueous sodium hydroxide, it gives sodium zincate and hydrogen gas.
The Why Questions
Electronic Configuration
Electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals.
Group 1 elements – Alkali metals
| Element | Electronic configuration |
| Lithium (Li) | 2,1 |
| Sodium (Na) | 2,8,1 |
| Potassium (K) | 2,8,8,1 |
| Rubidium (Rb) | 2,8,18,8,1 |
Group 2 elements – Alkaline earth metals
| Element | Electronic configuration |
| Beryllium (Be) | 2,2 |
| Magnesium (Mg) | 2,8,2 |
| Calcium (Ca) | 2,8,8,2 |
| Stronium (Sr) | 2,8,18,8,2 |
To know more about Electronic Configuration, visit here.
How Do Metals and Non-metals React
Metals lose valence electron(s) and form cations.
Non-metals gain those electrons in their valence shell and form anions.
The cation and the anion are attracted to each other by strong electrostatic force, thus forming an ionic bond.
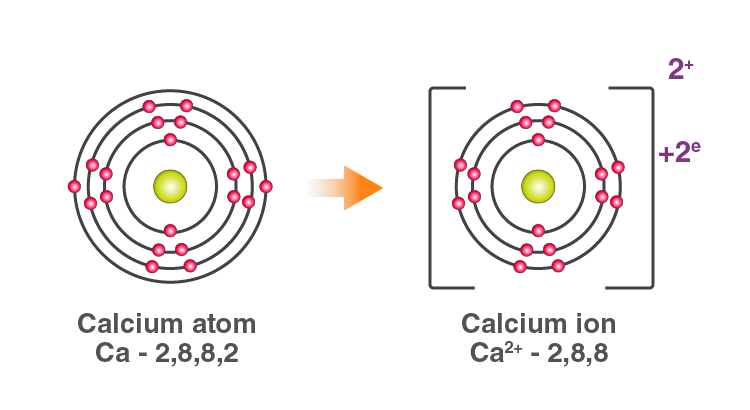
For example: In Calcium chloride, the ionic bond is formed by oppositely charged calcium and chloride ions.
Calcium atom loses 2 electrons and attains the electronic configuration of the nearest noble gas (Ar). By doing so, it gains a net charge of +2.
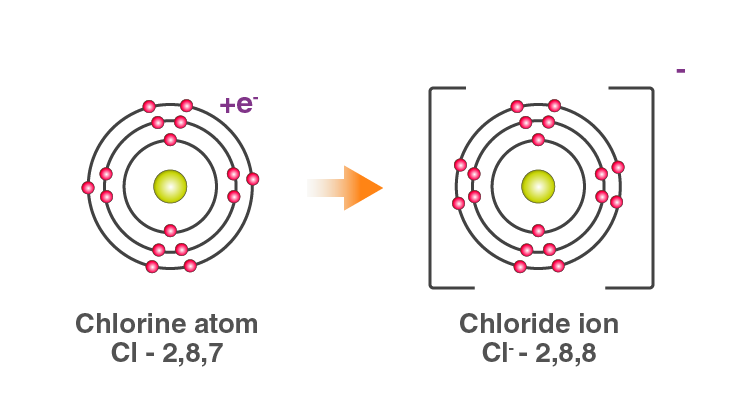
The two Chlorine atoms take one electron each, thus gaining a charge of -1 (each) and attain the electronic configuration of the nearest noble gas (Ar).
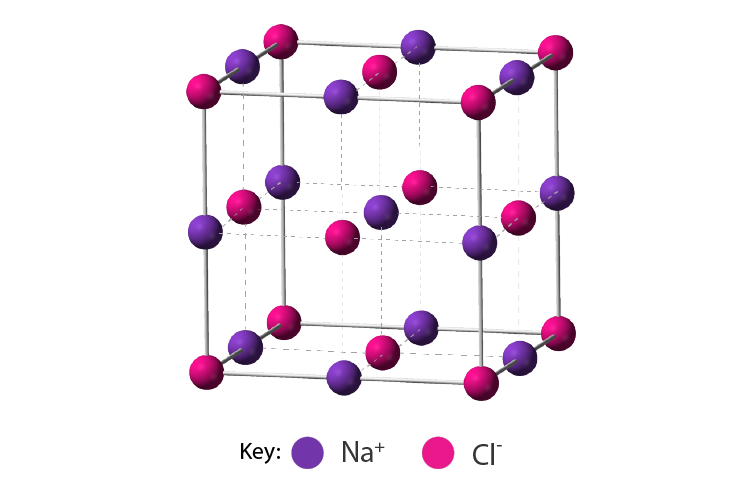
Ionic Compounds
- Ionic compounds are chemical compounds in which oppositely charged ions are held together by electrostatic forces called ionic bonds.
- An ionic compound always contains an equal magnitude of positive and negative charges. For example: CaCl2, NaCl, K2SO4, etc
Properties of Ionic Compounds
- Usually crystalline solids (made of ions).
- Have high melting and boiling points.
- Conduct electricity when in an aqueous solution or molten in water and when melted.
- Mostly soluble in water and polar solvents.
Physical Properties of Ionic Compounds
- Ionic compounds are solids and are hard to break due to the presence of a strong force of attraction between the positive and negative ions.
- They generally break into pieces when pressure is applied, hence are considered brittle.
Lattice Structure of Ionic Compounds
- A lattice is a regular arrangement of particles, whether these are atoms, ions or molecules.
- Ionic solids usually exist in regular, well-defined crystal structures.
Electric Conduction of Ionic Compounds
Ionic compounds conduct electricity in the molten or aqueous state when ions become free and act as charge carriers.
In solid form, ions are strongly held by electrostatic forces of attraction and not free to move; hence do not conduct electricity.
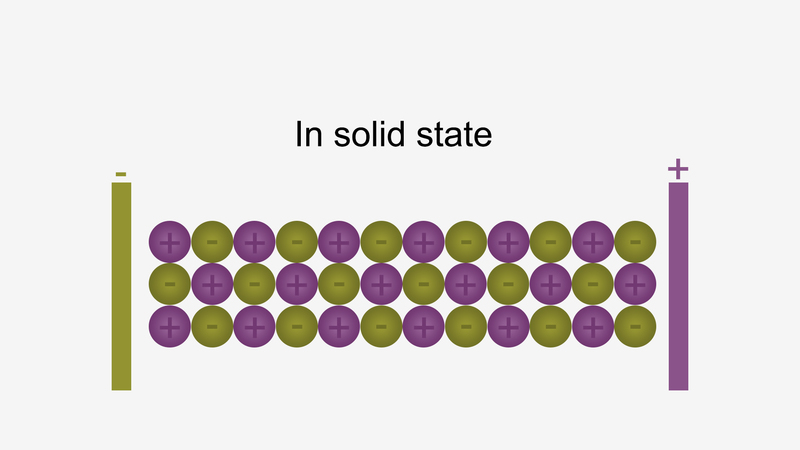
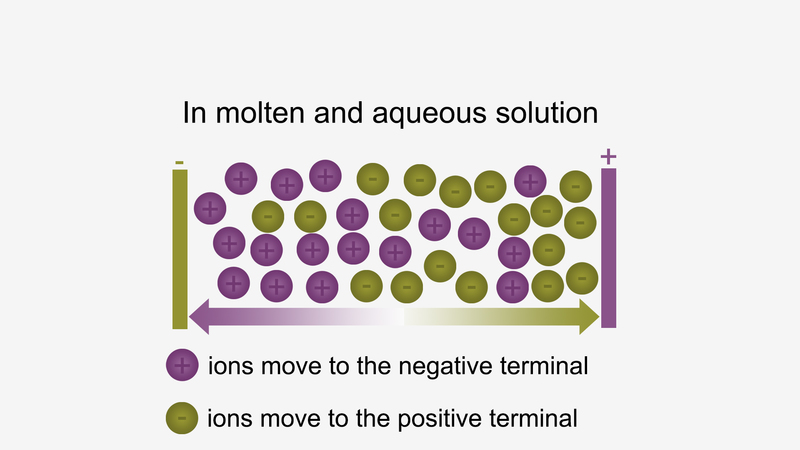
For example, ionic compounds such as NaCl do not conduct electricity when solid conduct electricity, but when dissolved in water or in a molten state, they will conduct electricity.
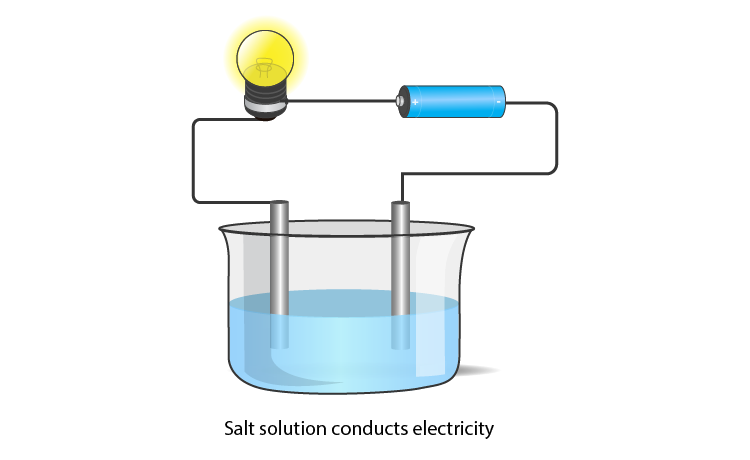
Melting and Boiling Points of Ionic Compounds
In ionic compounds, the strong electrostatic forces between ions require a high amount of energy to break. Thus, the melting point and boiling point of an ionic compound are usually very high.
Solubility of Ionic Compound
Ionic compounds are generally soluble in polar solvents such as water, whereas the solubility tends to decrease in non-polar solvents such as chloroform, oil, etc.
To know more about Ionic Compound, visit here.
Extraction of Metals and Non-Metals
Applications of Metals and Non-metals
- Zinc is used to protect the iron from rusting.
- Gold and silver are used for making jewellery.
- Oxygen is used by plants and animals.
- For the preparation of ammonia, nitric acid and fertilizers, nitrogen is used.
- For the purification of water, chlorine is used.
- Diamonds are used for cutting glass in different industries.
Occurrence of Metals
Most elements, especially metals, occur in nature in the combined state with other elements. All these compounds of metals are known as minerals. But out of them, only a few are viable sources of that metal. Such sources are called ores.
Au, Pt – exists in the native or free state.
Extraction of Metals
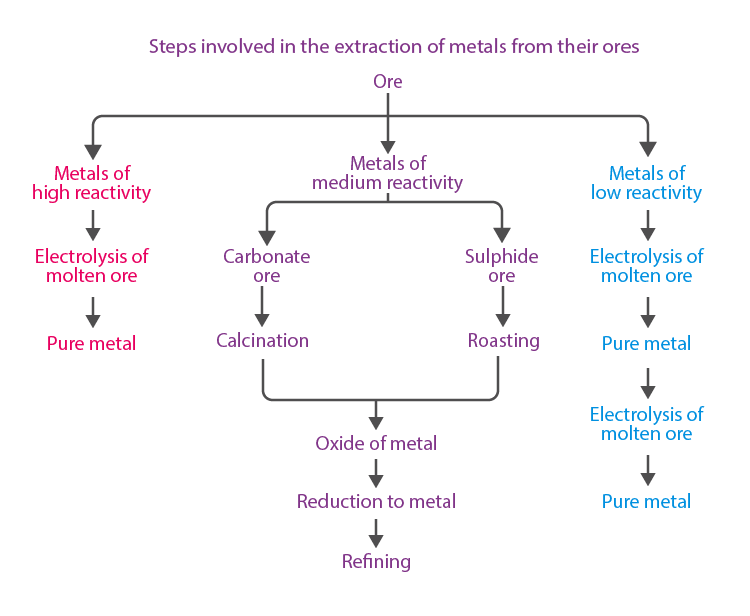
To know more about Extraction of Metals, visit here.
Roasting
Roasting:- Converts sulfide ores into metal oxides on heating strongly in the presence of excess air.
It also removes volatile impurities.
2ZnS(s)+3O2(g)+Heat→2ZnO(s)+2SO2(g)
Calcination
Calcination: Converts carbonate and hydrated ores into oxides on heating strongly in the presence of limited air. It also removes volatile impurities.
ZnCO3(s)+heat→ZnO(s)+CO2(g)
CaCO3(s)+heat→CaO(s)+CO2(g)
To know more about Roasting and Calcination, visit here.
Extraction of Metals, Which Are Lower in the Reactivity Series
- The metals like gold, silver, platinum, and copper are the least reactive and found in free state.
- Copper and silver are also found in the combined state as their sulphide or oxide ores. These metals usually occur as sulphide ores, which further undergo roasting.
Extraction of Metals, Which Are Mid-Way in the Reactivity Series
- Metals such as Zn, Fe, Pb, etc., are moderately reactive and usually present as oxides, sulphides or carbonates in nature.
- The carbonate and sulphide ores are subjected to calcination and roasting, respectively, and followed by a reduction of metal oxides to obtain the metals.
Extraction of Metals, Which Are on the Top of the Reactivity Series
- Na, Ca, Mg, Al, etc., cannot be obtained by reducing with C due to the high affinity for oxygen.
- These metals are obtained by electrolytic reduction or electrolysis of their oxides, hydroxides or chlorides in the molten state.
Enrichment of Ores
It means the removal of impurities or gangue from ore through various physical and chemical processes. The technique used for a particular ore depends on the difference in the properties of the ore and the gangue.
Refining of Metals
Refining of metals – removing impurities or gangue from crude metal. It is the last step in metallurgy and is based on the difference between the properties of metal and gangue.
Electrolytic Refining
- The process of purifying impure metal to obtain pure metal on the passage of electric current is called electrolytic refining.
- Metals like copper, zinc, nickel, silver, tin, gold etc., are refined electrolytically.
Anode – impure or crude metal
Cathode – a thin strip of pure metal
Electrolyte – aqueous solution of a metal salt From anode (oxidation) – metal ions are released into the solution
At cathode (reduction) – an equivalent amount of metal from the solution is deposited.
Impurities deposit at the bottom of the anode.
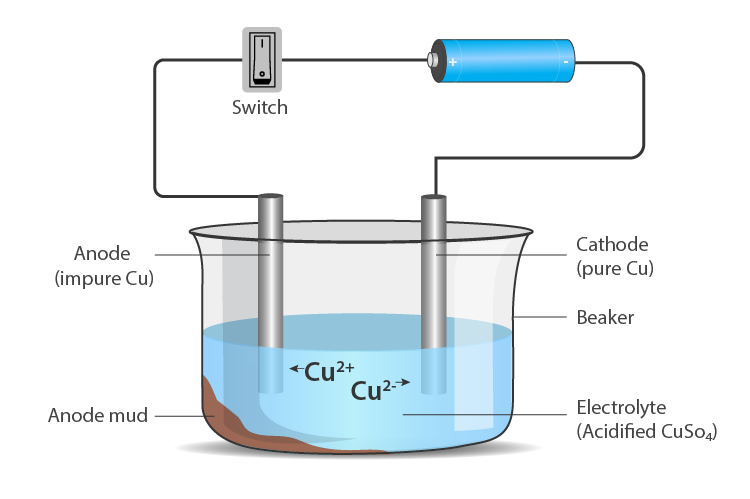
To know more about Electrolytic Refining, visit here.
| Also Access |
| NCERT Solutions for class 8 Science Chapter 4 |
| NCERT Exemplar for class 8 Science Chapter 4 |
Learn more about metals and non-metals and other related topics, including NCERT class 8 Science notes, at BYJU’S.
Also Read:
| Properties of Metals and Non-metals | Difference Between Metals and Non-metals |
Frequently Asked Questions on CBSE Class 8 Science Notes Chapter 4 Materials Metals and Non-Metals
What are Amphoteric oxide metals?
Amphoteric oxides are metal oxides which react with both acids as well as bases to form salt and water.
What is the Reactivity series?
The reactivity series of metals is a chart listing metals in order of decreasing reactivity.
What is a Lattice structure?
Lattice structures are topologically ordered, three-dimensional open-celled structures composed of one or more repeating unit cells.
Comments