
| Symbol | Am |
| Atomic Number | 95 |
| Atomic Mass | (243) |
| Discovered by | Americium was discovered by Glenn Seaborg and colleagues. |
Table of Contents
- Element information
- What is Americium?
- Production of Americium
- Properties of Americium
- Uses of Americium
- FAQs
Element Information
| Group | Actinides | Melting point | 1176°C, 2149°F, 1449 K |
| Period | 7 | Boiling point | 2011°C, 3652°F, 2284 K |
| Block | f | Density (g cm−3) | 12 |
| Atomic number | 95 | Relative atomic mass | [243] |
| State at 20°C | Solid | Key isotopes | 241Am, 243Am |
| Electron configuration | [Rn] 5f77s2 | CAS number | 7440-35-9 |
| ChemSpider ID | 22405 | ChemSpider is a free chemical database | |
What is Americium?
- Americium is a human-made actinide element part of the periodic table with an atomic number of 95 and has no stable isotopes.
- It was discovered by Glenn Seaborg, Leon Morgan, Ralph James, and Albert Ghiorso in 1944 and isolated by B.B. Cunningham as the isotope 241Am in Am(OH)3 in the fall of 1945.
- It was named after America’s (Seaborg 1991; Seaborg and Loveland 1990).
- Actinides are the 15 elements starting with actinium, atomic number 89, and extending to lawrencium, atomic number 103.
Production of Americium
- Americium has been produced in nuclear reactors for decades, It is synthesized from plutonium isotope 239Pu. In the nuclear reactors first Uranium converted to Plutonium isotope 239Pu , then Plutonium isotope 239Pu capture of two neutrons followed by beta decay result Americium – 241 (241Am).
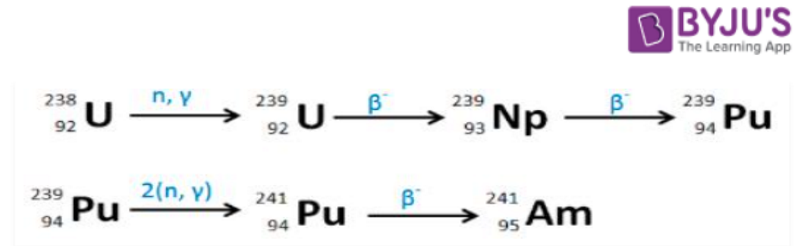
Properties of Americium
-
- The most stable oxidation state for americium is +3, it readily reacts with oxygen, hydrogen , halogen and many elements and dissolves in aqueous acids.
- Americium shows variable oxidation states such as +2, +3,+4,+5,+6,+7. Americium compounds with oxidation state +4 and higher are strong oxidizing agents.
- AmO2+2 is a strong oxidizer as KMnO4
- The color of americium compounds in aqueous solution is as follows:
Am3+ – yellow-reddish
Am4+ – yellow-reddish
AmO2+ – yellow
AmO2+2 – brown
AmO65− – dark green
Uses of Americium
- Americium-241 (241Am) is used in very small quantities in household ionization smoke detectors. Americium is similar to plutonium (Pu) in many ways. While the public accepts the use of minute quantities of 241Am in smoke detectors in their homes, the public reaction to transporting any quantity of 239Pu under suitable controls is very different.
- It plays a part in nuclear power production as a decay product.
- Due to the scarcity of Plutonium to make spacecraft batteries, Americium can serve as a viable replacement in the forthcoming years.
- Americium -241 is a valuable laboratory sources of alpha particles
Frequently Asked Questions – FAQs
What is americium used for?
Americium is a silvery, shiny radioactive metal. It is commonly used in smoke detectors, Due to the scarcity of Plutonium to make spacecraft batteries, Americium can serve as a viable replacement in the forthcoming years. It also used in laboratory as the sources of alpha particles.
How much americium is in a smoke detector?
The amount of americium used in a modern smoke detector is 1 microcurie (37 kBq). Some old industrial smoke detectors used up to 80 μCi.
What is the half life of americium 242?
The half life of Americium-242 is 16.02 hrs.
Why is americium used in smoke detectors?
Smoke detectors use americium as a source of alpha particles. Alpha particles from the americium source ionize air molecules form positively charged and some negatively charged particles. Two charged plates inside of the ionization smoke detector create a flow of positively and negatively charged ions. The smoke alarm triggers when smoke breaks the constant flow of ions.
What elements does americium react with?
Americium shows variable oxidation states such as +2, +3,+4,+5,+6, and +7. It readily reacts with oxygen, hydrogen, halogen and many elements to form various compounds. Americium reacts with oxygen to form AmO2 , it react with halogen form AmF4 and react with hydrogen form AmH2


Comments