A covalent bond is formed by the equal sharing of electrons from both participating atoms. The pair of electrons participating in this type of bonding is called a shared pair or bonding pair. Covalent bonds are also called molecular bonds. Sharing of bonding pairs will ensure that the atoms achieve stability in their outer shell, which is similar to the atoms of noble gases.
Download Complete Chapter Notes of Chemical Bonding and Molecular Structure
Download Now
JEE Main 2021 LIVE Chemistry Paper Solutions 24-Feb Shift-1 Memory-based

Table of Content
- Covalent Bonding in Carbon
- Properties
- Octet Rule
- Types of Covalent Bond
- Polarization of Covalent Bond
- Covalent vs Ionic Bonds
- Solved Examples
What Is Covalent Bond?
Elements having very high ionisation energies are incapable of transferring electrons, and elements having very low electron affinity cannot take up electrons. The atoms of such elements tend to share their electrons with the atoms of other elements or with other atoms of the same element in a way that both the atoms obtain octet configuration in their respective valence shells, and thus achieve stability. Such association through sharing of electron pairs among different or same kinds is known as Covalent Bond.

Formation of Covalent Bond
Covalent bonding can be achieved in two ways:
- Sharing of electrons between atoms of the same kind, for example, formation of H2, Cl2, O2, etc.
- Sharing of electrons between atoms of different kinds, for example, formation of CH4, H2O, NH3, etc.
Also Read:
Covalent Bonding in Carbon Atom
As per the electronic configuration of carbon, it needs to gain or lose 4 electrons to become stable, which seems impossible as:
- Carbon cannot gain 4 electrons to become C4-, because it will be tough for 6 protons to hold 10 electrons, and so the atom will become unstable.
- Carbon cannot lose 4 electrons to become C4+ because it would require a large amount of energy to remove out 4 electrons. Also, the C4+ would have only 2 electrons held by the proton, which will again become unstable.
Carbon cannot gain or donate electrons, so to complete its nearest noble gas configuration, it shares electrons to form a covalent bond.
Properties of Covalent Bond
If the normal valence of an atom is not satisfied by sharing a single electron pair between atoms, the atoms may share more than one electron pair between them. Some of the properties of covalent bonds are listed below:
- Covalent bonding does not result in the formation of new electrons. The bond only pairs them.
- They are very powerful chemical bonds that exist between atoms.
- A covalent bond normally contains an energy of about ~80 kilocalories per mole (kcal/mol).
- Covalent bonds rarely break spontaneously after it is formed.
- Covalent bonds are directional, where the atoms that are bonded showcase specific orientations relative to one another.
- Most compounds having covalent bonds exhibit relatively low melting points and boiling points.
- Compounds with covalent bonds usually have lower enthalpies of vaporisation and fusion.
- Compounds formed by covalent bonding don’t conduct electricity due to the lack of free electrons.
- Covalent compounds are not soluble in water.
What Is the Octet Rule?
All atoms except noble gases have less than eight electrons in their valence shell. In other words, the valence shells of these atoms do not have stable configurations. Therefore, they combine with each other or with other atoms to attain stable electronic configurations.
Therefore,
“The tendency of atoms of various elements to attain stable configuration of eight electrons in their valence shells is the cause of chemical combination”
and
“The principle of attaining the maximum of eight electrons in the valence shell of atoms is called the octet rule.”
Lewis introduced simple symbols to denote the electrons present in the outer shell of an atom known as the valence electrons. These symbols are known as Electron Dot Symbols, and the structure of the compound is known as Lewis Dot Structure.

Dot structure of methane
Conditions for Writing the Lewis Dot Structures
- Sharing of an electron pair between the atoms results in the formation of covalent bonds.
- During bond formation, each bond consists of two electrons which are contributed by each one of the combining atoms.
- By the mutual sharing of electrons, each atom attains an octet configuration in its valence shell.
Electron dot structures of covalent molecules are written with respect to the octet rule. According to this rule, all the atoms in the molecule will have eight electrons in their valence shell except the hydrogen atom. Hydrogen will have only two electrons because only two electrons complete its first shell to attain helium configuration.
Thus the elements of group 17, such as Cl, would share one electron to attain a stable octet; the elements of group 16, such as O and S, would share two electrons; the elements of group 15 would share three electrons and so on.
For Example, the oxygen atom, which has six electrons in its valence shell, completes its octet by sharing its two electrons with two hydrogen atoms to form a water molecule.

Lewis Structure of Water Molecule
Types of Covalent Bonds
Depending upon the number of shared electron pairs, the covalent bond can be classified into:
- Single Covalent Bond
- Double Covalent Bond
- Triple Covalent Bond
Single Bonds
A single bond is formed when only one pair of electrons is shared between the two participating atoms. It is represented by one dash (-). Although this form of covalent bond has a smaller density and is weaker than a double and triple bond, it is the most stable.
For example, the HCL molecule has one hydrogen atom with one valence electron and one chlorine atom with seven valence electrons. In this case, a single bond is formed between hydrogen and chlorine by sharing one electron.
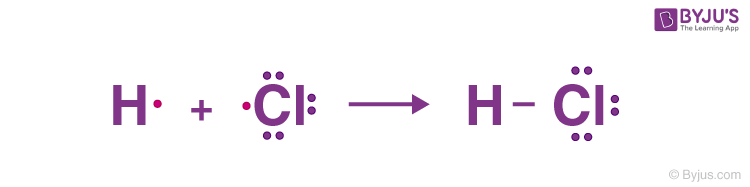
Double Bonds
A double bond is formed when two pairs of electrons are shared between the two participating atoms. It is represented by two dashes (=). Double covalent bonds are much stronger than single bonds, but they are less stable.
For example, a carbon dioxide molecule has one carbon atom with six valence electrons and two oxygen atoms with four valence electrons.
To complete its octet, carbon shares two of its valence electrons with one oxygen atom and two with another oxygen atom. Each oxygen atom shares its two electrons with carbon, and therefore there are two double bonds in CO2.

CO2 Molecule with Double Covalent Bond
Oxygen Molecule: In the formation of the oxygen molecule, each oxygen atom has six electrons in its valence shell. Each atom requires two more electrons to complete its octet. Therefore, the atoms share two electrons each to form the oxygen molecule. Since two electron pairs are shared, there is a double bond between the two oxygen atoms.

O2 Molecule with Double Covalent Bond
Ethylene Molecule: In ethylene, each carbon atom shares two of its valence electrons with two hydrogen atoms and the remaining two electrons with the other carbon atom. So, there is a double bond between the carbon atoms.

Double Bond in Ethylene Molecule
Triple Bond
A triple bond is formed when three pairs of electrons are shared between the two participating atoms. Triple covalent bonds are represented by three dashes (≡) and are the least stable type of covalent bonds.
For example, in the formation of a nitrogen molecule, each nitrogen atom having five valence electrons provides three electrons to form three electron pairs for sharing. Thus, a triple bond is formed between the two nitrogen atoms.

Nitrogen Molecule with Triple Bond
Polar Covalent Bond
This type of covalent bond exists where the unequal sharing of electrons occurs due to the difference in the electronegativity of combining atoms. More electronegative atoms will have a stronger pull for electrons. The electronegative difference between the atoms is greater than zero and less than 2.0. As a result, the shared pair of electrons will be closer to that atom.
For example, molecules form hydrogen bonding as a result of an unbalanced electrostatic potential. In this case, the hydrogen atom interacts with electronegative fluorine, hydrogen, or oxygen.
Nonpolar Covalent Bond
This type of covalent bond is formed whenever there is an equal share of electrons between atoms. The electronegativity difference between two atoms is zero. It occurs wherever the combining atoms have similar electron affinity (diatomic elements).
For example, Nonpolar Covalent Bond is found in gas molecules like hydrogen gas, nitrogen gas, etc.
Polarization of Covalent Bonds
It is observed that in the sigma bonds between two different atoms, the electron cloud is always closer to the more electronegative of the two atoms participating in the sigma bond. Due to this, there is a permanent dipole that arises in the bond, and the covalent bond is said to be polarized.

Polarity of Covalent Bond in Water Molecule
An illustration describing the polarity of the covalent bonds in a water molecule is provided above. The more electronegative atom is said to have a partial negative charge, and the less electronegative atom has a partial positive charge in the polar covalent bond.
Difference between Covalent and Ionic Bonds
Covalent bonds and ionic bonds are types of atomic bonds. These bonds are different in their properties and structure. Covalent bonds include pairs of electrons by two atoms binding them in a fixed orientation, while a bond between two ions is called an ionic bond.

Covalent vs Ionic Bonds
Covalent bonding occurs between two non-metallic atoms, characterised by the sharing of electron pairs between the atoms and other covalent bonds with an electronegativity difference greater than 2.0 (<2.0). In the case of covalent bond formation, polyatomic ions are formed, whereas the ionic bond is formed as a result of electrostatic attraction between the oppositely charged ions.
Ionic Bond vs Covalent Bond
|
Difference between Ionic and Covalent Bond |
|
|
Covalent Bonds |
Ionic Bonds |
| A covalent bond is formed between two similar electronegative non-metals | This type of bond is formed between a metal and non-metal |
| Bonds formed from covalent bonding have a definite shape | Ionic bonds have no definite shape |
| Low melting point and boiling point | High melting point and boiling point |
| Low polarity and more flammable | High polarity and less flammable |
| Covalent bonds are in a liquid or gaseous state at room temperature | At room temperature, ionic bonds have a solid state. |
| Examples: Methane, Hydrochloric acid | Examples: Sodium chloride, Sulfuric Acid |
Also, Check ⇒ Difference between Ionic, Covalent and Metallic Bonds
The presence of a bond between two elements can be determined by calculating the electronegative value between two atoms.
| Bond Type | Electronegativity Value |
| Polar Covalent Bond | 0.5 to 1.9 |
| Non-polar Covalent Bond | 0 to 0.4 |
| Ionic Bond | 2.4 to 4.0 |
Solved Examples
1. Which of the following compounds contains both covalent and ionic bonds?
a. NaOH
b. NaBr
c. NaNC
d. NaCN
Answer: (c) NaNC
A covalent bond is present between N and C Atom, and an ionic bond is present between the Na+ ion and –NC ion.
2. A chemical bonding between the two atoms which shares a single pair of an electron is
a. Ionic bond
b. Single bond
c. Double bond
d. Triple bond
Answer: (b) Single bond
3. Which of the following compounds contains both polar and non-polar covalent bonds?
a. NH4Br
b. H2O2
c. CH4
d. HF
Answer: (b) H2O2
In H2O2, the electronegativity difference between o and H atoms is 1.4, so O – H bond is polar.
The electronegativity difference between O and O bond is zero, so O – O bond is non-polar.
4. Draw the Lewis structure of:
- Carbon Tetrachloride (CCl4)
- Ammonia (NH3)
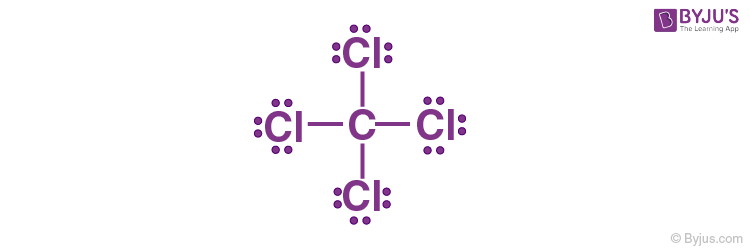
1. Carbon has four electrons in its valence shell and completes its octet by sharing its four electrons with four chlorine atoms to form a carbon tetrachloride (CCl4) molecule, as shown below.
2. Nitrogen has five electrons in its valence shell and completes its octet by sharing its three electrons with three hydrogen atoms to form NH3 (Ammonia).

Lewis Structure of Ammonia (NH3)
Introduction to Chemical Bonding

Chemical Bonding

Comments