What are Radioactive elements?
Some elements of atomic nuclei are unstable because of the presence of excess nuclear charge inside it, so these nuclei undergo radioactive decay to form stable nuclei. These elements are called radioactive elements.
The stability of nuclei of an element can be calculated by n/p (neutron to proton ratio). Elements of atomic number up to Z= 20 are stable and contain an equal number of protons and neutrons. As the atomic number increases, the repulsion between protons increases and requires more protons and neutrons. Thus, the neutron to proton ratios of stable nuclei increases with increasing atomic number.
Elements having atomic number up to Z = 20 , n/p =1.0 . And the elements having atomic number up to Z=83, n/p = 1.5 . If elements have the atomic number, Z > 83, n/p will be greater than 1.5 and it will be most unstable and hence radioactive.
Table of contents
- Radioactive element decay process
- List of radioactive elements
- Application of radioactive elements
- Frequently Asked Questions – FAQs
Radioactive element decay process
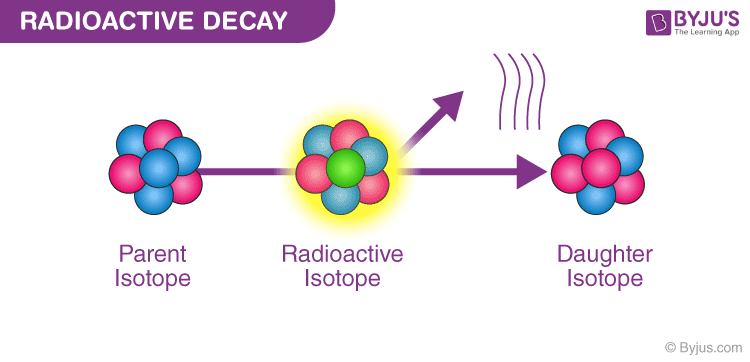
1. Alpha decay:
When an alpha particle emits its nucleus, the process is called alpha decay. The formula of alpha decay is given as:
The nucleus of helium is taken as the alpha particle which is very stable. It has a group of two protons and two neutrons. For example, the alpha decay of uranium-238 is shown below
238U92 → 234Th90 + 4He2
- An Alpha particle is composed of 2 protons and 2 neutrons.
- It has symbols 4He2
- They have high ionisation energy power.
- Alpha particle has low penetrating power
2. Beta Decay
A beta particle is often referred to as an electron, but it can also be a positron. If the reaction involves electrons, the nucleus sheds out neutrons one by one. Even the proton number increases accordingly. A beta decay process is shown below:
234Th90 → 234Pa91 + e-10
- Beta particles are electrons emitted from atomic nuclei
- It has symbols e-10
- They have intermediate ionisation energy power.
- The beta particle has intermediate penetrating power
3. Gamma Decay
The nucleus has orbiting electrons which indeed have some energy, and when an electron jumps from a level of high energy to a level of low energy, there is an emission of a photon. The same thing happens in the nucleus: whenever it rearranges into a lower energy level, a high-energy photon is shot out which is known as a gamma ray.
238U92 → 234Th90 + 4He2 + γ00
- Gamma rays are electromagnetic radiation high-energy photons.
- It has symbols γ00
- They have low ionization energy power.
- Gamma rays have a high penetrating power.
List of radioactive elements in the periodic table
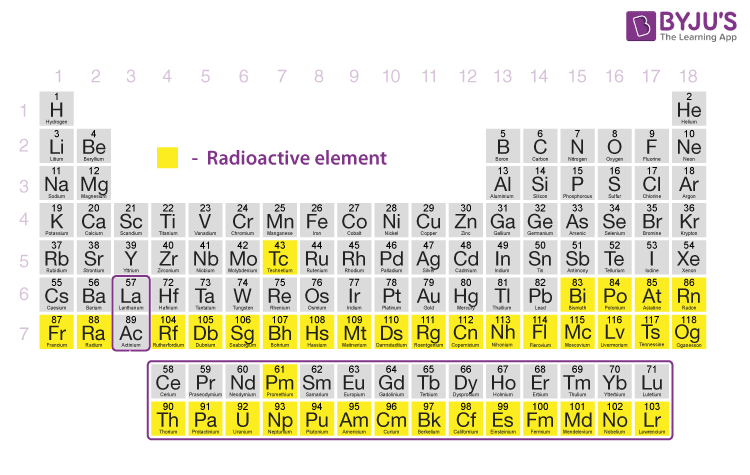
Here’s the list of radioactive elements and their most stable isotopes:
| Radioactive Elements | Atomic number | Most stable isotope |
|---|---|---|
| Technetium | 43 | Tc-97 |
| Promethium | 61 | Pm-145 |
| Polonium | 84 | Po-209 |
| Astatine | 85 | At-210 |
| Radon | 86 | Rn-222 |
| Francium | 87 | Fr-223 |
| Radium | 88 | Ra-226 |
| Actinium | 89 | Ac-227 |
| Thorium | 90 | Th-232 |
| Protactinium | 91 | Pa-231 |
| Uranium | 92 | U-235 |
| Neptunium | 93 | Np-237 |
| Plutonium | 94 | Pu-244 |
| Americium | 95 | Am-243 |
| Curium | 96 | Cm-248 |
| Berkelium | 97 | Bk-247 |
| Californium | 98 | Cf-251 |
| Einsteinium | 99 | Es-252 |
| Fermium | 100 | Fm-257 |
| Mendelevium | 101 | Md-258 |
| Nobelium | 102 | No-259 |
| Lawrencium | 103 | Lr-266 |
| Rutherfordium | 104 | Rf-267 |
| Dubnium | 105 | Db-268 |
| Seaborgium | 106 | Sg-269 |
| Bohrium | 107 | Bh-278 |
| Hassium | 108 | Hs-269 |
| Meitnerium | 109 | Mt – 282 |
| Darmstadtium | 110 | Ds-281 |
| Roentgenium | 111 | Rg-286 |
| Copernicium | 112 | Cn-285 |
| Nihonium | 113 | Nh-286 |
| Flerovium | 114 | Fl-290 |
| Moscovium | 115 | Mc-290 |
| Livermorium | 116 | Lv-293 |
| Tennessine | 117 | Ts-294 |
| Oganesson | 118 | Og-294 |
Application of radioactive elements
- Radioactive isotopes have many useful applications. In medicine, it is used for cancer treatment and also used as tracers for diagnostic purposes as well as in research on metabolic processes
- Gamma rays are used to kill cancerous cells and are hence used in radiotherapy.
- Cobalt-60 is used to destroy carcinogenic cells.
- I-131 is used for thyroid function tests and for treating thyroid cancer.
- Sr-89, used for treating bone cancer, intravenous injection.
- Gamma rays kill microbes present in food and prevent it from decaying by increasing the shelf life.
- The age of the rocks can be studied using radioactive radiations by measuring the argon content present in the rock
Frequently Asked Questions on Radioactive elements
What is a radioactive element?
Some elements of atomic nuclei are unstable because of the presence of excess nuclear charge inside it so these nuclei undergo radioactive decay to form stable nuclei. These elements are called radioactive elements.
What are the 4 radioactive elements?
The common 4 radioactive elements are Uranium, Radium, Polonium, Thorium etc.
Are smokers’ lungs radioactive?
Cigarettes made from tobacco contain radioactive materials: polonium-210 and lead-210. These radioactive particles settle in smokers’ lungs, where they build up as long as the person smokes. Over time, the radiation can damage the lungs and can contribute to lung cancer. They also harm people exposed to secondhand smoke.
What is uranium used for?
U-235 and U-238 occur naturally in nearly all rock, soil, and water. Uranium is now used to power commercial nuclear reactors that produce electricity and to produce isotopes used for medical, industrial, and defence purposes around the world.
What are the examples of radioactive waste?
Radioactive waste is a type of hazardous waste that contains radioactive material. Any activity related to the nuclear fuel cycle that produces or uses radioactive materials generates radioactive waste. The activities like mining and processing of uranium ore, fabrication of nuclear fuel, generation of power in nuclear reactors, production and use of radionuclides for various industrial and medical applications, research associated with radioactive material etc. generate the different types of radioactive waste.
Comments