
| Symbol | Ne |
| Atomic Number | 10 |
| Atomic Mass | 20.180 |
| Discovered by | Neon was discovered by Sir William Ramsay and Morris Travers |
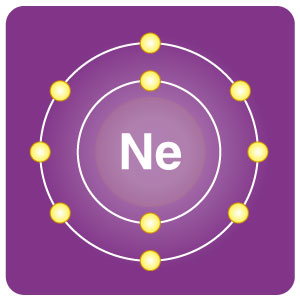
Table of contents
What is Neon?
- Neon is reddish-orange coloured in neon lamps and vacuum discharge tubes and the second-lightest noble gas. It is less expensive refrigerant than helium in many applications. Its refrigerating capacity is 40 times more than liquid helium and three times of liquid hydrogen as per unit volume basis. It is a rare gas, and its molecules consist of a single Neon atom.
- It is a chemically inert gas and non-toxic in nature. There is no threat to the environment and has no impact since it’s non-reactive and does not form compounds. This element cause no ecological damage.
- It can create exotic compounds with fluorine in laboratories, being an inert element. It also forms an unstable hydrate.
Suggested Videos

Chemical Properties of Neon
| Group | 18 | Melting point | −248.59°C, −415.46°F, 24.56 K |
| Period | 2 | Boiling point | −246.046°C, −410.883°F, 27.104 K |
| Block | p | Density (g cm−3) | 0.000825 |
| Atomic number | 10 | Relative atomic mass | 20.180 |
| State at 20°C | Gas | Key isotopes | 20Ne |
| Electron configuration | [He] 2s22p6 | CAS number | 7440-01-9 |
| ChemSpider ID | 22377 | ChemSpider is a free chemical structure database | |
Uses of Neon
- The reddish-orange coloured neon lights are used in making advertising signs. It’s also utilized in these types of lights generally when many other gases are needed to generate lights of different colours.
- Other uses of neon include lightning arrestors, high-voltage indicators, television tubes and meter tubes.
- Gas lasers are made with the help of neon and helium.
- The electronics industry uses neon singly or in mixtures with other gases in many types of gas-filled electron tubes.
- A mixture of helium and neon is used for respiration by marine divers in the sea since helium is less soluble in blood than nitrogen at high pressure.
Frequently Asked Questions – FAQs
What is the Colour of neon?
The colour of neon is orange-red when placed in an electric field.
What is neon gas used for?
Neon lights are used as arrestors, high-voltage indicators, television tubes, and meter tubes. Neon lamps are used in botanical gardens and also used in filling sodium vapour lamps.
What are 5 interesting facts about neon?
(a) Neon is the rarest element on Earth.
(b) Neon is the fifth most abundant element in the universe.
(c) It produces an orange-red glow, when placed in an electric field.
(d) Neon is mainly used in fluorescent lamps.
(e) It is known as Neon sign and is used for advertising purposes, it can be seen at long distances even when there is fog.
Is neon gas expensive?
Neon is a rare gas so it’s expensive to produce. It is about 55 times more expensive than liquid helium.
How was neon gas discovered?
Neon gas was discovered in 1898 by British chemists: Sir William Ramsay and Morris W. Travers. They chilled a sample of air until it became a liquid, then warmed this liquid, capturing the gases as they boiled off. After removing the gases which had already been identified. They saw brilliant red light under spectroscopic discharge. This was neon.

Comments