Group 14 elements of the modern periodic table are also known as carbon group elements. These elements have 4 valence electrons in their outermost shell. The compounds formed by these elements play an important role in the existence of life on earth.
The members of group 14 are:
- Carbon
- Silicon
- Germanium
- Lead
- Tin
Table of Contents
The uses of the oxides of group 14 elements are mentioned below.
Carbon monoxide

Carbon monoxide is a colourless and odourless toxic gas with the chemical formula CO. It is a flammable gas, which is used in the manufacturing of various inorganic and organic chemicals. Uses of carbon monoxide are:
- Carbon monoxide(CO) is industrially a very important gas, as it is used in the manufacturing of various organic and inorganic compounds.
- It is used in modified atmosphere packaging systems to keep packaged meat fresh.
- In high-power infrared lasers, it is used as a lasing medium.
Carbon dioxide
Carbon dioxide is colourless and odourless gas in nature. It has the chemical formula CO2. It plays a vital role in the existence of life on the earth. This gas is found in traces in the atmosphere but it is of great importance.

Some common uses of carbon dioxide are given below:
- Carbon dioxide finds its application in a large number of industries such as oil industries, food industries, and chemical industries.
- It is used in the manufacturing of urea in various chemical industries.
- In food industries, it is used as a food additive.
- It is used in fire extinguishers.
Silicon dioxide
![]()
Silicon dioxide (SiO2) is one of the chemical compounds known since medieval times. Silicon dioxide is also called silica. Silica is found abundantly in the earth’s crust. Uses of silica are:
- Silica is mainly used in the production of glass, which is used for windows, bottles, etc.
- It is a major ingredient in sand casting, which is responsible for the manufacture of a large number of engineering components and other materials.
- It is used in the food industry as a common additive.
Silicones
Silicones are polymers which are made up of repeating units of siloxane. Siloxane is made up of alternating oxygen and silicon atoms. These polymers are heat-resistant.
![]()
Uses of silicones are:
- It is used as a lubricant in the automotive industry.
- Silicones are used in the coating of silica-based substrates.
- It is used in deformers as an active compound due to its good spreading properties and low solubility in water.
Zeolites
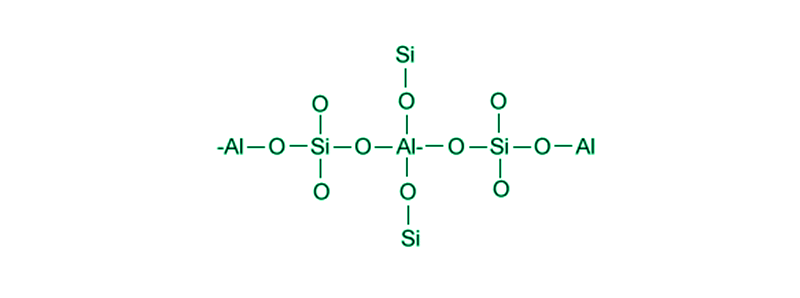
Zeolites are solid structures which are crystalline in nature. They are made up of aluminium, silicon, and oxygen. Their structure consists of cavities where water molecules, and cations, can reside. They are also called molecular sieves. These minerals are mined extensively due to their application in a large number of fields. Some of their applications are mentioned below:
- It is used in photochemical industries as a catalyst.
- Zeolites are used in nuclear industries due to their microporous ability.
- It is used in the manufacturing of detergents.
The compounds of carbon group elements have been an essential part of human development and evolution.
Carbon dioxide plays an important role in photosynthesis in which plants and some bacteria use energy from the sun to produce glucose. The glucose produced gets converted into pyruvate which further releases adenosine triphosphate (ATP) by cellular respiration.
Even though carbon monoxide is widely used it is a poisonous gas.
Silica, silicones, and silicates can be called as an important class of compounds owing to their diverse applicability.
For any doubt regarding the uses of oxides of group 14 elements get in touch with the mentor support team at BYJU’S.

Comments