
| Symbol | N |
| Atomic Number | 7 |
| Atomic Mass | 14.007 |
| Discovered by | Daniel Rutherford in 1772 |

Chemical Properties of Nitrogen
| Group | 15 | Melting point | −210.0°C, −346.0°F, 63.2 K |
| Period | 2 | Boiling point | −195.795°C, −320.431°F, 77.355 K |
| Block | p | Density (g cm−3) | 0.001145 |
| Atomic number | 7 | Relative atomic mass | 14.007 |
| State at 20°C | Gas | Key isotopes | 14N |
| Electron configuration | [He] 2s22p3 | CAS number | 7727-37-9 |
| ChemSpider ID | 20473555 | ChemSpider is a free chemical structure database | |
What is Nitrogen?
- The seventh element of the periodic table between carbon and oxygen is nitrogen.
- It’s an important part of amino acids.
- Around eighty per cent of the Earth’s atmosphere comprises nitrogen gas.
- It has no colour, mostly diatomic non-metal gas which is odourless and colourless in nature.
- Since it has five electrons in its outer shell, most of its compounds are trivalent.
- It is a constituent of all living tissues. Since it is a component of DNA and part of a genetic code, it is an essential element of life.
- It is found in nitrates and nitrites in soil and water.
- All these substances are part of the nitrogen cycle and interconnected. Industrial companies emit nitrogen extensively, increasing nitrite and nitrate content in the ground and water, being the consequence of reactions in the nitrogen cycle.
Nitrogen Uses
- It is used in the manufacture of ammonia, to produce nitric acid and subsequently used as a fertilizer.
- Nitric acid salts include important compounds like potassium nitrate, ammonium nitrate, and nitric acid. Nitrated organic compounds such as nitro glycerine are often explosives.
- Liquid nitrogen is utilized as a refrigerant for transporting foodstuff and freezing purposes. Preservation of bodies and reproductive cells and stable storage of biological samples also makes use of liquid nitrogen.
- Nitrogen makes up 78 per cent of the Earth’s atmosphere and is a part of all living tissue. Nitrogen is a crucial ingredient of life since it is a constituent of DNA and as such is part of the genetic code.
- Nitrogen molecules often exist in the soil. Nitrogen can be present in nitrates and nitrites in water and in soil. These compounds are all part of the nitrogen cycle and both are interconnected.
Nitrogen Cycle
A cycle is a sequence of events or steps that repeats itself regularly. In the nitrogen cycle, nitrogen moves from the soil to plants and then to animals and finally back to the soil. When it returns to the soil from a decaying plant it can be used again by another plant.
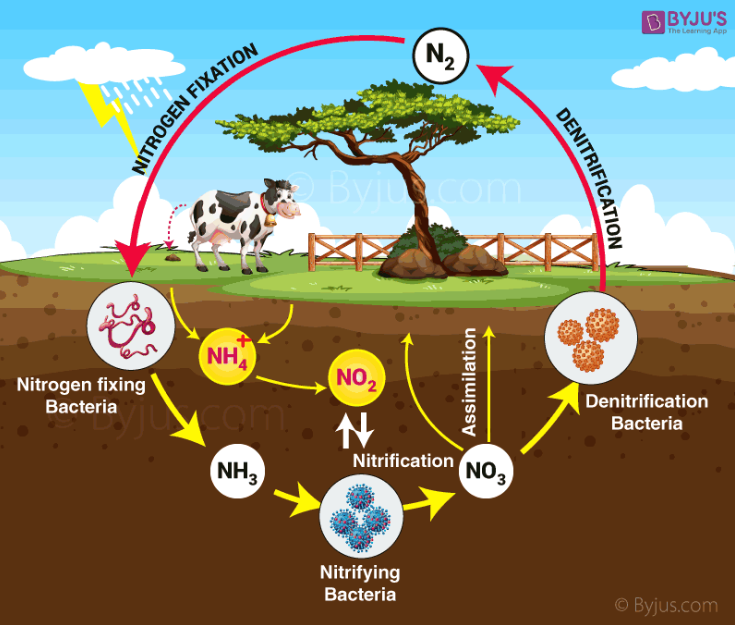 The nitrogen cycle has five general steps.
The nitrogen cycle has five general steps.
- Nitrogen fixation
- Nitrification
- Denitrification
- Nitrogen assimilation
- Ammonification.
Read More: Nitrogen Cycle
Over many years the actions of people began changing how nitrogen cycled through nature. This changed the amount of nitrogen found in living organisms and in the air, soil, and water. The balance of nature was upset. By understanding how the nitrogen cycle works people can change their actions and protect the environment.
Recommended Videos

Frequently Asked Questions – FAQs
Where is nitrogen found?
It is the fifth most abundant element in the universe, making up about 78 per cent of the atmosphere on earth, and contains an estimated 4,000 trillion tons of gas. Nitrogen is extracted through a process called fractional distillation from liquefied air.
Why is nitrogen used??
Nitrogen is used as an effective way to prevent oxidation and provides a safe, inert atmosphere which “sweeps” off furnace-generated gases. This is also used as a laser cutting assist steam, that facilitates plasma cutting. Nitrogen is used in a broad variety of applications for upstream and midstream electricity.
How do you fix nitrogen?
A wide range of microorganisms called diazotrophs, including bacteria such as azotobacter, and archaea, naturally conduct nitrogen fixation in the soil. Some nitrogen-fixing bacteria, particularly legumes, have symbiotic relationships with certain plant groups.
What would happen if nitrogen-fixing bacteria did not exist?
Bacteria transform airborne nitrogen and carbon dioxide into functional components that can be used as basic building blocks by plants and animals. To living organisms, a loss of all microbes would be terrible news that they can not produce or receive such essential nutrients on their own.
How do plants take up nitrogen?
In the form of nitrate (NO3−) and ammonium (NH4 +), plants absorb nitrogen from the soil. Nitrate is typically the predominant type of absorbed nitrogen available in aerobic soils where nitrification can occur.


Comments