What is Atomic Theory?
Dalton’s atomic theory was a scientific theory on the nature of matter put forward by the English physicist and chemist John Dalton in the year 1808. It stated that all matter was made up of small, indivisible particles known as ‘atoms’.
All substances, according to Dalton’s atomic theory, are made up of atoms, which are indivisible and indestructible building units. While an element’s atoms were all the same size and mass, various elements possessed atoms of varying sizes and masses.
Table of Contents
- Postulates of Dalton’s Atomic Theory
- Limitations of Dalton’s Atomic Theory
- What are the Merits of Dalton’s Atomic Theory?
- Recommended Videos
- Frequently Asked Questions – FAQs
Postulates of Dalton’s Atomic Theory
- All matter is made up of tiny, indivisible particles called atoms.
- All atoms of a specific element are identical in mass, size, and other properties. However, atoms of different element exhibit different properties and vary in mass and size.
- Atoms can neither be created nor destroyed. Furthermore, atoms cannot be divided into smaller particles.
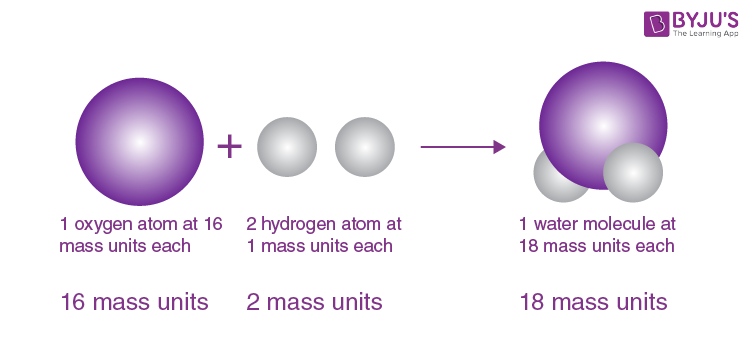
- Atoms of different elements can combine with each other in fixed whole-number ratios in order to form compounds.
- Atoms can be rearranged, combined, or separated in chemical reactions.
Limitations of Dalton’s Atomic Theory
- It does not account for subatomic particles: Dalton’s atomic theory stated that atoms were indivisible. However, the discovery of subatomic particles (such as protons, electrons, and neutrons) disproved this postulate.
- It does not account for isotopes: As per Dalton’s atomic theory, all atoms of an element have identical masses and densities. However, different isotopes of elements have different atomic masses (Example: hydrogen, deuterium, and tritium).
- It does not account for isobars: This theory states that the masses of the atoms of two different elements must differ. However, it is possible for two different elements to share the same mass number. Such atoms are called isobars (Example: 40Ar and 40Ca).
- Elements need not combine in simple, whole-number ratios to form compounds: Certain complex organic compounds do not feature simple ratios of constituent atoms. Example: sugar/sucrose (C11H22O11).
- The theory does not account for allotropes: The differences in the properties of diamond and graphite, both of which contain only carbon, cannot be explained by Dalton’s atomic theory.
Dalton’s Atomic Theory – The Indestructible Atoms
What are the Merits of Dalton’s Atomic Theory?
- The law of multiple proportions, the law of conservation of mass, and the law of constant proportions are not violated by Dalton’s atomic theory.
- The theory provides a basis to differentiate between elements and compounds.
Recommended Videos
The Periodic Table

Atomic Structure

To learn more about Dalton’s atomic theory and other related concepts such as Thomson’s atomic model, register with BYJU’S and download the mobile application on your smartphone.
Frequently Asked Questions – FAQs
How does Dalton’s atomic theory explain the law of conservation of mass?
Since it states that atoms cannot be created or destroyed, Dalton’s theory suggests that the net mass of the participating species in a chemical reaction is conserved. This postulate, therefore, accounts for the law of conservation of mass.
How does Dalton’s atomic theory differentiate between elements and compounds?
This theory states that elements combine in fixed, whole-number ratios to form compounds. Therefore, it suggests that compounds are made up of molecules that contain two or more atoms of different elements.
What are the 5 key postulates of Dalton’s atomic theory?
The 5 postulates of Dalton’s atomic theory are listed below.
- All matter is made up of atoms, which are tiny, indivisible particles.
- All the atoms of an element have the same size, mass, and properties but the atoms of different elements have different sizes and masses.
- Atoms cannot be created, destroyed, or divided into smaller particles.
- Compounds are formed when the atoms of different elements combine with each other in fixed, whole-number ratios.
- Atoms can be combined, separated, or rearranged via chemical reactions.
List two merits of Dalton’s atomic theory.
One of the most important merits of Dalton’s atomic theory is the fact that the theory does not violate several fundamental laws of chemical combination such as the law of definite proportions, the law of multiple proportions, and the law of conservation of mass. Another important merit of Dalton’s atomic theory is that it provided a basis for scientists to differentiate between elements and compounds.
What are the shortcomings of Dalton’s atomic theory?
Some important demerits of Dalton’s atomic theory are listed below.
- The theory did not account for the existence of subatomic particles (it suggested that atoms are indivisible).
- By suggesting that all atoms of an element must have identical masses and sizes, Dalton’s atomic theory did not account for the existence of isotopes. Furthermore, this theory also did not account for the existence of isobars (nuclides of different chemical elements with the same mass number).
- Dalton’s atomic theory failed to explain the dissimilarities in the properties of different allotropes of an element.
- This theory states that elements must combine in simple, whole-number ratios to form compounds. However, this is not necessarily true. Several complex organic compounds do not feature simple ratios of their constituent elements.
Do electrons actually exist?
Most of us realize that the neutron, in an atom of matter, is a negatively charged particle orbiting the nucleus. No two electrons at the same time will occupy the same space. They are part of any molecule, but they may also live on their own, independently.
Which atomic model is used today?
The Bohr paradigm, generally speaking, encapsulates the popular understanding of the atom. In artwork that depicts a central atomic nucleus and oval lines reflecting the electron orbits, this image is also portrayed.

Good explanation of questions
It helps to understand the question answer and explain the topic
What is the correction of Dalton’s Atomic theory?
The indivisibility of an atom was proved wrong: an atom can be further subdivided into protons, neutrons and electrons. However, an atom is the smallest particle that takes part in chemical reactions. According to Dalton, the atoms of same element are similar in all respects.