Nernst Equation
Trending Questions
When sodium acetate is added to aqueous solution of acetic acid, then why does the ph value increase?
Given: E0(Zn2+|Zn)=−0.76V, E0(Cu2+Cu)=−0.34V
There are two types of concentration cells.
(i) Electrode concentration Cell: In this type of cell electrode has different concentration of electrode material in the same electrolyte.
Ex. Pt|H2(P1 atm)|HCl(cM)|H2(P2 atm)|Pt
(ii) Electrolyte Concentration Cell: In this type of cell, same electrode is immersed in the electrolyte having common species but different concentrations.
Ex. Pt|H2(1 atm)|HCl(c1M)||HCl(c2M)|H2 (1 atm)|Pt
Q. Calculate emf of the following concentration cell at 25°C.
Pt|H2(1 atm)|HCl(10–5 M)||CH3COOH(0.1 M)|H2(1 atm)|Pt
[Given: Ka (CH3COOH) = 10–5. Use 2.3030RTF=0.06]
नीचे दिए गए प्रश्न के लिए अनुच्छेद
सांद्रता सेल दो प्रकार के होते हैं :
(i) इलेक्ट्रॉड सांद्रता सेल: इस प्रकार के सेल में, एक ही विद्युतअपघटय में स्थित इलेक्ट्रॉडों के पदार्थ की सांद्रता भिन्न होती है।
उदाहरण : Pt|H2(P1 atm)| HCl(cM)|H2(P2 atm)|Pt
(ii) विद्युतअपघट्य सांद्रता सेल: इस प्रकार के सेल में समान इलेक्ट्रॉड, समान स्पीशीज लेकिन भिन्न सांद्रताओं वाले विद्युत अपघट्य में डूबे रहते हैं।
उदाहरण : Pt|H2(1 atm)|HCl(c1M)|| HCl(c2M)|H2 (1 atm)| Pt
प्रश्न. 25°C पर निम्नलिखित सांद्रता सेल के वि.वा.ब. (emf) की गणना कीजिए
Pt|H2(1 atm)|HCl(10–5 M)||CH3COOH(0.1 M)|H2(1 atm)|Pt
[दिया गया है: Ka(CH3COOH) = 10–5, प्रयोग करें : 2.3030RTF=0.06]
- 0.12 V
- 0.18 V
- 0.03 V
- 0.06 V
There are two types of concentration cells.
(i) Electrode concentration Cell: In this type of cell electrode has different concentration of electrode material in the same electrolyte.
Ex. Pt|H2(P1 atm)|HCl(cM)|H2(P2 atm)|Pt
(ii) Electrolyte Concentration Cell: In this type of cell, same electrode is immersed in the electrolyte having common species but different concentrations.
Ex. Pt|H2(1 atm)|HCl(c1M)||HCl(c2M)|H2 (1 atm)|Pt
Q. EMF of the following electrochemical cell Pt|H2(1 atm)|HA(0.1 M)||HCl(0.1 M)|H2(1 atm)|Pt is 0.18 V at 25°C. Then calculate Ka of weak acid HA. (UseRT×2.303F=0.06]
नीचे दिए गए प्रश्न के लिए अनुच्छेद
सांद्रता सेल दो प्रकार के होते हैं :
(i) इलेक्ट्रॉड सांद्रता सेल: इस प्रकार के सेल में, एक ही विद्युतअपघटय में स्थित इलेक्ट्रॉडों के पदार्थ की सांद्रता भिन्न होती है।
उदाहरण : Pt|H2(P1 atm)| HCl(cM)|H2(P2 atm)|Pt
(ii) विद्युतअपघट्य सांद्रता सेल: इस प्रकार के सेल में समान इलेक्ट्रॉड, समान स्पीशीज लेकिन भिन्न सांद्रताओं वाले विद्युत अपघट्य में डूबे रहते हैं।
उदाहरण : Pt|H2(1 atm)|HCl(c1M)|| HCl(c2M)|H2 (1 atm)| Pt
प्रश्न. 25°C पर विद्युत रासायनिक सेल Pt|H2(1 atm)|HA(0.1 M)||HCl(0.1 M)|H2(1 atm)|Pt का वि.वा.ब. (EMF) 0.18 V है। तब दुर्बल अम्ल HA के Ka की गणना कीजिए।
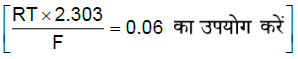
- 2 × 10–5
- 5 × 10–6
- 1 × 10–8
- 1 × 10–7
Pb2+aq+2e−→Pb(s) E0=−0.121VPbO2(s)+4H+(aq)+SO2−4(aq)+2e−→PbSO4(s)+2H2O E0=1.71VPb4+(aq)+2e−→Pb2+(aq) E0=1.689V
For the cell PbSO4(s)+2e−→Pb(s)+SO2−4(aq),
The E0 is missing but it is found that in an aqueous solution of H2SO4 having a pH=1.7, when PbSO4(s) is put, then at saturated condition
Solubility of PbSO4=5×10−5Mol/L
(Take 2.303RTF=0.06 and log2=0.3)
List-I contains questions and List-II contains their answers.
List-IList-IIWhat is the E0(in V) for the half-cell(I) PbSO4(s)+2e−→Pb(s)+SO2−4(aq)?(P) -0.168What is the E0cell(in V) of a lead storage battery? It has(II) the cell reaction asPb(s)+PbO2(s)+2H2SO4(aq)→2PbSO4(s)+2H2O(l)(Q) 0.7What is the E0(in V) for the half cell(III)PbO2(s)+4H+(aq)+4e−→Pb(s)+2H2O(I)(R) 2.0Ksp is the equilibrium constant for the reaction(IV) PbO2(s)+4H+(aq)⇌Pb4+(aq)+2H2O(l)(S) -0.068What is the value of Eo (in V)?(T) -0.31(U) 2.02
Which of the following options has the correct combination considering the reactions in List-I and their answers in List-II?
- (II), (R) and (IV), (S)
- (II), (U) and (IV), (S)
- (II), (R) and (IV), (P)
- (II), (U) and (IV), (P)
- 10−10atm
- 10−4atm
- 10−14atm
- 10−12atm
- P1>P2
- 2P1<P2
- P1=P2
- 2P2>P1
- the amount of metal in the anode
- the size of the electrode
- the Eo values of the half-cells
- the pH of the solution
2Fe(s)+O2(g)+4H+(aq)→2Fe2+(aq)+2H2O(l) ; E∘=1.67 V
At [Fe2+]=10−3M, P(O2)=0.1 atm and pH = 3, the cell potential at 25 ∘C is
(given: 2.303RTF=0.0591)
One day Mr.Nernst told her to find the EMF of the following cell using experiments at 25∘C
Mg(s)+2Ag+(0.0001M)→Mg2+(0.130M)+2Ag(s)
Electra reported the value to be +2.6V. Mr.Nernst told her to recheck the value using his theory, knowing Standard EMF = 3.17 V.
What was the right value? Round off the answer to nearest integer.
Type your answer here.
Electra has trouble finding the correct Nernst equation for the cell Zn(s)|Zn2+||Cu2+|Cu(s) at 25∘C
Can you help her?
Ecell=E∘−0.0296 log([Zn2+][Cu2+])
Ecell=E∘−0.0296 log([Cu2+][Zn2+])
Ecell=E∘−0.0296 log(ZnCu)
Ecell=E∘−0.0296 log(CuZn)
Zn|ZnSO4 (0.01M)|| CuSO4(1.0M)Cu, the emf of this Daniell cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the followings, which one is the relationship between E1 and E2? (Given, RT/F = 0.059)
- E1<E2
- E1>E2
- E2=−E1
- E1=E2
Pb2+aq+2e−→Pb(s) E0=−0.121VPbO2(s)+4H+(aq)+SO2−4(aq)+2e−→PbSO4(s)+2H2O E0=1.71 VPb4+(aq)+2e−→Pb2+(aq) E0=1.689V
For the cell PbSO4(s)+2e−→Pb(s)+SO2−4(aq),
The E0 is missing but it is found that in an aqueous solution of H2SO4 having a pH=1.7, when PbSO4(s) is put, then at saturated condition
Solubility of PbSO4=5×10−5Mol/L
(Take 2.303RTF=0.06 and log2=0.3)
List-I contains questions and List-II contains their answers.
List-IList-IIWhat is the E0(in V) for the half-cell(I) PbSO4(s)+2e−→Pb(s)+SO2−4(aq)?(P) -0.168What is the E0cell(in V) of a lead storage battery? It has(II) the cell reaction asPb(s)+PbO2(s)+2H2SO4(aq)→2PbSO4(s)+2H2O(l)(Q) 0.7What is the E0(in V) for the half cell(III)PbO2(s)+4H+(aq)+4e−→Pb(s)+2H2O(I)(R) 2.0Ksp is the equilibrium constant for the reaction(IV) PbO2(s)+4H+(aq)⇌Pb4+(aq)+2H2O(l)(S) -0.068What is the value of Eo (in V)?(T) -0.31(U) 2.02
Which of the following options has the correct combination considering the reactions in List-I and their answers in List-II?
- (I), (S) and (III), (P)
- (I), (T) and (III), (Q)
- (I), (Q) and (III), (R)
- (I), (U) and (III), (R)
- −(E1+E2)2×0.059
- (E1+E2)2×0.059
- (E2−E1)2×0.059
- −(E1+E2)0.059
(a) CrO3
(b) Fe2O3
(c) MnO2
(d) V2O5
(e) Cu2O
JEE MAIN 2021
[ E0Cu2+/Cu= 0.34 V, E0Zn2+/Zn= -0.76 V ]
The emf of the cell increases when small amount of concentrated NH3 is added to:
- ZnSO4 solution
- CuSO4 solution
- Both ZnSO4 and CuSO4
- Can't say
Pt|H2(P1 atm)|H+(M1)||H+(M2)|H2(P2 atm)|Pt
where P1 and P2 are pressures. M1 and M2 are molarities .
What will be the emf of cell at 25∘C If P1=P2 and M1 is 50% higher than M2 ?
Repeat the magnitude of emf by multiplying with 10000.
[Take : 2.303RTF=0.06and log 3=0.48, log2=0.3]
Electra has trouble finding the correct Nernst equation for the cell Zn(s)|Zn2+||Cu2+|Cu(s) at 25∘C
Can you help her?
Ecell=E∘−0.0296 log([Zn2+][Cu2+])
Ecell=E∘−0.0296 log([Cu2+][Zn2+])
Ecell=E∘−0.0296 log(ZnCu)
Ecell=E∘−0.0296 log(CuZn)
Pt(s)|H2(g, 1bar)|H+(aq, 1M)||M4+(aq), M2+(aq)|Pt(s)
Ecell=0.092V when[M2++(aq)][M4+(aq)]=10x
Given:E∘M4+/M2+=0.151V;2.303RTF=0.059V
The value of x is
- -2
- -1
- 1
- 2
- Zn(s)|Zn2+(aq)(0.02M)||H3O+(aq)(0.1M)|H2(g)(0.5atm), Pt
- Cu(s)|Cu2+(aq)(0.25M)||Ag+(aq)(0.5M)|Ag
- Cd(s)|Cd2+(aq)(0.01M)||H+(pH=1)|H2(g)(1atm), Pt
- Zn(s)|Zn2+(aq)(0.1M)||H+(pH=1)|H2(g)(1atm), Pt
The Edison storage cell is represented as :
Fe(s)/FeO(s)/KOH(aq)/Ni2O3 (s)/Ni(s)
The half-cell reactions are:
Ni2O3(s)+H2O(l)+2e−→2NiO(s)+2OH−;E∘+0.40
FeO(s)+H2O(l)+2e−→Fe(s)+2OH−;E∘=−0.87,
What is the maximum amount of electrical energy that can be obtained from one mole of Ni2O3 ?
344.6 KJ
87.9 KJ
2478.8 KJ
245.11 KJ
Calculate the emf of the cell
Pt, H2(1.0atm) | CH3COOH (0.1M) || NH3(aq, 0.001M) | H2(1.0 atm), Pt Ka(CH3COOH) = 10−5, Kb(NH3) = 10−5
+0.368 V
-0.413 V
-0.233 V
+0.567 V
The e.m.f. of the cell at 25∘C
CuCu2+(0.01M)||Ag+(0.1M)Ag
is [E0(Cu2+Cu)=0.34V and E0(Ag+Ag)=0.80V]
0.46V
1.14V
0.43V
1.11V
One day Mr.Nernst told her to find the EMF of the following cell using experiments at 25∘C
Mg(s)+2Ag+(0.0001M)→Mg2+(0.130M)+2Ag(s)
Electra reported the value to be +2.6V. Mr.Nernst told her to recheck the value using his theory, knowing Standard EMF = 3.17 V.
What was the right value? Round off the answer to nearest integer.
Type your answer here.
