1
Question
A metal carbonate X on reacting with an acid gives a gas which when passed through a solution gives the carbonate black. On the other hand, A gas G that is obtained at anode during electrolysis of brine is passed on dry Y, it gives compound Z, used for disinfecting water. Identify X, Y, G and Z.
Open in App
Solution
According to the problem:Here,X is calcium carbonate (CaCO3).Y is slaked lime.
Z is bleaching powder (CaOCl2)G is chlorine gas.
Hence the reactions are involved, 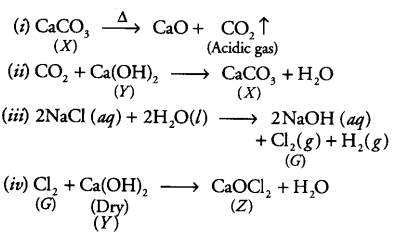
Here,
X is calcium carbonate (CaCO3).
Y is slaked lime.
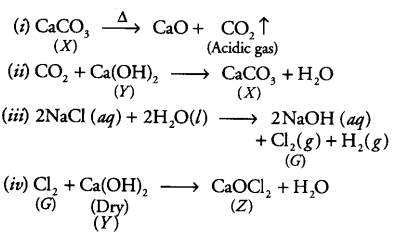
Z is bleaching powder (CaOCl2)
G is chlorine gas.
Hence the reactions are involved,
Suggest Corrections
0
View More
Join BYJU'S Learning Program
Join BYJU'S Learning Program
