A solid body of constant heat capacity
1 J∘C is being heated by keeping it in contact with reservoirs in two ways:
(i) Sequentially keeping in contact with 2 reservoirs such that each reservoir supplies same amount of heat.
(ii) Sequentially keeping in contact with 8 reservoirs such that each reservoirs supplies same amount of heat in both cases the body is brought from initial temperature 100∘C to final temperature 200∘C. Entropy change of the body in two cases respectively is :
A solid body of constant heat capacity
1 J∘C is being heated by keeping it in contact with reservoirs in two ways:
(i) Sequentially keeping in contact with 2 reservoirs such that each reservoir supplies same amount of heat.
(ii) Sequentially keeping in contact with 8 reservoirs such that each reservoirs supplies same amount of heat in both cases the body is brought from initial temperature 100∘C to final temperature 200∘C. Entropy change of the body in two cases respectively is :
The correct option is B In2,
It is
the type of matter which has got fixed shape and volume .The force of
attraction between any two molecules of a solid is very large .
- wherein
e.g.Nacl,Diamond,Graphite
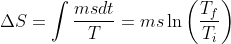
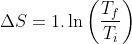
△Sis state depedent. Its value changes by changing state hence if remain same in two processes.
△S =In2
It is the type of matter which has got fixed shape and volume .The force of attraction between any two molecules of a solid is very large .
- wherein
e.g.Nacl,Diamond,Graphite
△Sis state depedent. Its value changes by changing state hence if remain same in two processes.
△S =In2
